LBF20406AM38: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&N- (2-methyl-2-hydroxyethyl) arachidonoylamide (S)&& | |Common Name=&&N- (2-methyl-2-hydroxyethyl) arachidonoylamide (S)&& | ||
|Melting Point=colorless oil [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | |Melting Point=colorless oil [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
| | |Optical=dX<sub>4</sub><sup>25</sup>= +9.44° [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (200 MHz, CDCl3) <FONT FACE="Symbol">d</FONT>(TMS)6.42 (m, 1H), 5.46-5.30 (m, 8H), 3.93-3.85 (m, 1H), 3.47-3.36 (m, 1H), 3.16-3.03 (m, 1H), 2.83-2.80 (m, 6H), 2.26-2.01 (m, 6H), 1.78-1.64 (m, 2H), 1.39-1.25 (m, 6H), 1.18 (d, J=3.18Hz, 3H), 0.89 (t, J=6.43 Hz, 3H) [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (200 MHz, CDCl3) <FONT FACE="Symbol">d</FONT>(TMS)6.42 (m, 1H), 5.46-5.30 (m, 8H), 3.93-3.85 (m, 1H), 3.47-3.36 (m, 1H), 3.16-3.03 (m, 1H), 2.83-2.80 (m, 6H), 2.26-2.01 (m, 6H), 1.78-1.64 (m, 2H), 1.39-1.25 (m, 6H), 1.18 (d, J=3.18Hz, 3H), 0.89 (t, J=6.43 Hz, 3H) [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
|Chromatograms=Rf 0.3(5% MeOH/CHCl3) | |Chromatograms=Rf 0.3(5% MeOH/CHCl3) | ||
| Line 15: | Line 15: | ||
|Chemical Synthesis=This compound was synthesized from arachidonic acid and (S)-(+)-l-amino-2-propanol in 63% yield. [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | |Chemical Synthesis=This compound was synthesized from arachidonic acid and (S)-(+)-l-amino-2-propanol in 63% yield. [[Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889|{{RelationTable/GetFirstAuthor|Reference:Abadji_V:Lin_S:Taha_G:Griffin_G:Stevenson_LA:Pertwee_RG:Makriyannis_A:,J. Med. Chem.,1994,37,1889}}]] | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity={{Image200|LBF20406AM38FT7055.gif}} [[Reference:Seltzman_HH:Fleming_DN:Thomas_BF:Gilliam_AF:McCallion_DS:Pertwee_RG:Compton_DR:Martin_BR:,J. Med. Chem.,1997,40,3626|{{RelationTable/GetFirstAuthor|Reference:Seltzman_HH:Fleming_DN:Thomas_BF:Gilliam_AF:McCallion_DS:Pertwee_RG:Compton_DR:Martin_BR:,J. Med. Chem.,1997,40,3626}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 21:00, 6 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR7055 |
| LipidMaps | LMFA08020041 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406AM38 |
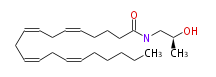
| N- (2-methyl-2-hydroxyethyl) arachidonoylamide (S) | |
|---|---|
| |
| Structural Information | |
| N- (2-methyl-2-hydroxyethyl) arachidonoylamide (S) | |
| |
| Formula | C23H39NO2 |
| Exact Mass | 361.298079497 |
| Average Mass | 361.5613 |
| SMILES | C(CCC=CCC=CCC=CCC=CCCCCC)C(NC[C@H](C)O)=O |
| Physicochemical Information | |
| colorless oil Abadji_V et al. | |
| dX425= +9.44° AbadjiVet al. | |
| This compound was synthesized from arachidonic acid and (S)-(+)-l-amino-2-propanol in 63% yield. Abadji_V et al. | |
 Seltzman_HH et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (200 MHz, CDCl3) d(TMS)6.42 (m, 1H), 5.46-5.30 (m, 8H), 3.93-3.85 (m, 1H), 3.47-3.36 (m, 1H), 3.16-3.03 (m, 1H), 2.83-2.80 (m, 6H), 2.26-2.01 (m, 6H), 1.78-1.64 (m, 2H), 1.39-1.25 (m, 6H), 1.18 (d, J=3.18Hz, 3H), 0.89 (t, J=6.43 Hz, 3H) AbadjiVet al. |
| Other Spectra | |
| Chromatograms | Rf 0.3(5% MeOH/CHCl3) |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|