LBF20406CV01: Difference between revisions
No edit summary |
No edit summary |
||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR8035 | |LipidBank=XPR8035 | ||
|LipidMaps=LMFA03120016 | |LipidMaps=LMFA03120016 | ||
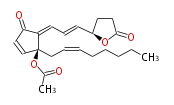
|SysName= | |SysName=4R- { (trans-1,trans-3) -3- [2S-Acetoxy-2- (cis-2-octenyl) -5-oxo-3-cyclopentenylidene] -1-propenyl} -4-butanolide | ||
|Common Name=&& | |Common Name=&&Clavulolactone II&&4R- { (1E,3E) -3- [2S-Acetoxy-2- (2Z-octenyl) -5-oxo-3-cyclopentenylidene] -1-propenyl} -4-butanolide&& | ||
| | |Optical=[ alpha ]_D -25.6°(C 0.26, CHCl_3 )[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|Mass Spectra=EIMS m/z 372 (M | |Mass Spectra=EIMS m/z 372 (M^+ ). HREIMS m/z 372.1920 for C_{22}H_{28}O_5 , calcd 372.1937.[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|UV Spectra= | |UV Spectra= lambda ^{EtOH}_{max} 292 nm( epsilon 16500),231 nm( epsilon 12500)[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|IR Spectra= | |IR Spectra= nu ^{film}_{max}1778,1745, 1704, 1644, and 1231cm^{-1}[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR(400MHz,CDCl_3 ) delta ppm0.87(3H,t,J=7.2Hz),1.20-1.34(6H,m),1.94(2H,brq,J=6.3Hz),2.02(3H,s),2.04(1H,m),2.52(1H,m),2.55(2H,m),2.71(1H,brdd,J=8.1,14.2Hz),2.91(1H,brdd,J=7.1,14.2Hz),5.15(1H,m),5.15(1H,m),5.52(1H,m),6.17(1H,dd,J=4.9,14.7Hz),6.42(1H,d,J=6.1Hz),6.82(1H,ddd,J=1.5,12.0,14.7Hz),6.91(1H,brd,J=12.0Hz),7.50(1H,brd,J=6.1Hz).[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] ^{13}C-NMR(100MHz,CDCl_3 ) delta ppm14.0,21.2,22.5,27.4,27.7,28.2,29.0,31.5,35.8,78.6,85.2,121.0,124.9,128.9,135.06,135.14,137.2,140.8,158.0,169.3,176.2,193.3.[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | ||
|Source=Clavulolactones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard.[[Reference:Iwashima_M:Okamoto_K:Konno_F:Iguchi_K:,J. Nat. Prod.,1999,62,352|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Konno_F:Iguchi_K:,J. Nat. Prod.,1999,62,352}}]][[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | |||
|Chemical Synthesis=Clavulolactone II was converted from clavulone II.[[Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790|{{RelationTable/GetFirstAuthor|Reference:Iguchi_K:Iwashima_M:Watanabe_K:,J. Nat. Prod.,1995,58,790}}]] | |||
|Metabolism= | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 05:49, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8035 |
| LipidMaps | LMFA03120016 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV01 |
| Clavulolactone II | |
|---|---|
| |
| Structural Information | |
| 4R- { (trans-1,trans-3) -3- [2S-Acetoxy-2- (cis-2-octenyl) -5-oxo-3-cyclopentenylidene] -1-propenyl} -4-butanolide | |
| |
| Formula | C22H28O5 |
| Exact Mass | 372.193674006 |
| Average Mass | 372.45472000000007 |
| SMILES | CC(=O)O[C@@](CC=CCCCCC)(C=2)C(C(C2)=O)=CC=C[C@H](O1)CCC(=O)1 |
| Physicochemical Information | |
| [ α ]D -25.6°(C 0.26, CHCl3) IguchiKet al. | |
| Clavulolactones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iwashima_M et al. Iguchi_K et al. | |
| Clavulolactone II was converted from clavulone II. Iguchi_K et al. | |
| Spectral Information | |
| Mass Spectra | EIMS m/z 372 (M+). HREIMS m/z 372.1920 for C22}H_{28O5, calcd 372.1937. IguchiKet al. |
| UV Spectra | λ EtOH max 292 nm( ε 16500),231 nm( ε 12500) IguchiKet al. |
| IR Spectra | ν film max 1778,1745, 1704, 1644, and 1231cm-1 IguchiKet al. |
| NMR Spectra | 1H-NMR(400MHz,CDCl3) δ ppm0.87(3H,t,J=7.2Hz),1.20-1.34(6H,m),1.94(2H,brq,J=6.3Hz),2.02(3H,s),2.04(1H,m),2.52(1H,m),2.55(2H,m),2.71(1H,brdd,J=8.1,14.2Hz),2.91(1H,brdd,J=7.1,14.2Hz),5.15(1H,m),5.15(1H,m),5.52(1H,m),6.17(1H,dd,J=4.9,14.7Hz),6.42(1H,d,J=6.1Hz),6.82(1H,ddd,J=1.5,12.0,14.7Hz),6.91(1H,brd,J=12.0Hz),7.50(1H,brd,J=6.1Hz). IguchiKet al. 13C-NMR(100MHz,CDCl3) δ ppm14.0,21.2,22.5,27.4,27.7,28.2,29.0,31.5,35.8,78.6,85.2,121.0,124.9,128.9,135.06,135.14,137.2,140.8,158.0,169.3,176.2,193.3. IguchiKet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|