LBF20406CV03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 8: | Line 8: | ||
|SysName=methyl (4R,5E,7E) -4-acetoxy-7- [ (R) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -5-heptenoate | |SysName=methyl (4R,5E,7E) -4-acetoxy-7- [ (R) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -5-heptenoate | ||
|Common Name=&&4-epiclavulone II&&methyl (4R,5E,7E) -4-acetoxy-7- [ (R) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -5-heptenoate&& | |Common Name=&&4-epiclavulone II&&methyl (4R,5E,7E) -4-acetoxy-7- [ (R) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -5-heptenoate&& | ||
| | |Optical=[<FONT FACE="Symbol">a</FONT>]<SUB><FONT SIZE=-1>D</FONT></SUB> -18.7°(C 0.30, CHCl<SUB><FONT SIZE=-1>3</FONT></SUB>)[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | ||
|Mass Spectra=EIMS m/z 446 (M<SUP><FONT SIZE=-1>+</FONT></SUP>). HREIMS m/z 446.2315 for C<SUB><FONT SIZE=-1>2</FONT></SUB><SUB><FONT SIZE=-1>5</FONT></SUB>H<SUB><FONT SIZE=-1>3</FONT></SUB><SUB><FONT SIZE=-1>4</FONT></SUB>O<SUB><FONT SIZE=-1>7</FONT></SUB> (M<SUP><FONT SIZE=-1>+</FONT></SUP>), calcd 446.2305.[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | |Mass Spectra=EIMS m/z 446 (M<SUP><FONT SIZE=-1>+</FONT></SUP>). HREIMS m/z 446.2315 for C<SUB><FONT SIZE=-1>2</FONT></SUB><SUB><FONT SIZE=-1>5</FONT></SUB>H<SUB><FONT SIZE=-1>3</FONT></SUB><SUB><FONT SIZE=-1>4</FONT></SUB>O<SUB><FONT SIZE=-1>7</FONT></SUB> (M<SUP><FONT SIZE=-1>+</FONT></SUP>), calcd 446.2305.[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | ||
|UV Spectra=<FONT FACE="Symbol">l</FONT><SUP><FONT SIZE=-1>E</FONT></SUP><SUP><FONT SIZE=-1>t</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> 228 nm(log <FONT FACE="Symbol">e</FONT>4.28),291 nm(log <FONT FACE="Symbol">e</FONT>4.27)[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | |UV Spectra=<FONT FACE="Symbol">l</FONT><SUP><FONT SIZE=-1>E</FONT></SUP><SUP><FONT SIZE=-1>t</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> 228 nm(log <FONT FACE="Symbol">e</FONT>4.28),291 nm(log <FONT FACE="Symbol">e</FONT>4.27)[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | ||
Revision as of 21:00, 6 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8042 |
| LipidMaps | LMFA03120023 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV03 |
| 4-epiclavulone II | |
|---|---|
| |
| Structural Information | |
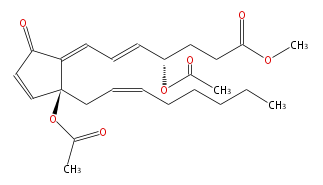
| methyl (4R,5E,7E) -4-acetoxy-7- [ (R) -2-acetoxy-2- [ (Z) -2-octenyl ] -5-oxo-3-cyclopentenylidene ] -5-heptenoate | |
| |
| Formula | C25H34O7 |
| Exact Mass | 446.230453442 |
| Average Mass | 446.53326000000004 |
| SMILES | O(C(C)=O)[C@@](C1=CC=C[C@H](CCC(OC)=O)OC(C)=O)(CC=CCCCCC)C=CC1=O |
| Physicochemical Information | |
| [a]D -18.7°(C 0.30, CHCl3) IwashimaMet al. | |
| 4-Epiclavulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iwashima_M et al. | |
| 4-Epiclavulone II was synthesized from clavulone II. Iwashima_M et al. | |
| Spectral Information | |
| Mass Spectra | EIMS m/z 446 (M+). HREIMS m/z 446.2315 for C25H34O7 (M+), calcd 446.2305. IwashimaMet al. |
| UV Spectra | lEtOHmax 228 nm(log e4.28),291 nm(log e4.27) IwashimaMet al. |
| IR Spectra | nfilmmax1738,1732,1704,1644,and 1232cm-1 IwashimaMet al. |
| NMR Spectra | 1H-NMR(500MHz,CDCl3)dppm0.88(3H,t,J=7.2Hz),1.20-1.34(6H,m),1.94(2H,q,J=6.8Hz),1.98-2.06(2H,m),2.03(3H,s),2.09(3H,s),2.37(2H,t,J=7.4Hz),2.71(1H,dd,J=8.4,14.1Hz),2.98(1H,dd,J=7.0,14.1Hz),3.67(3H,s),5.18(1H,ddd,J=7.0,8.4,10.9Hz),5.43(1H,dt,J=6.4,7.0Hz),5.50(1H,dt,J=7.4,10.9Hz),6.07(1H,dd,J=6.4,11.9Hz),6.41(1H,d,J=6.1Hz),6.70(1H,ddd,J=1.0,11.9,15.1Hz),6.88(1H,d,J=15.1Hz),7.48(1H,d,J=6.1Hz). IwashimaMet al. 13C-NMR(125MHz,CDCl3)dppm14.0,20.9,21.2,22.5,27.4,29.1,29.2,29.6,31.5,35.9,51.8,72.7,85.3,121.1,126.0,129.4,135.1,135.1,136.7,141.5,157.9,169.2,169.9,172.9,193.4. IwashimaMet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|