LBF20406CV04: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=XPR8043 | |LipidBank=XPR8043 | ||
|LipidMaps=LMFA03120024 | |LipidMaps=LMFA03120024 | ||
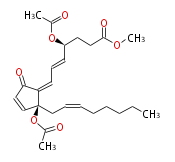
|SysName=( | |SysName=(4S,12S) - Bis(acetyloxy)-9-oxoprosta- (5-cis,7-cis,10-cis,13-cis) -tetrene-1-oic acid methyl ester | ||
|Common Name=&&4-epi Clavulone | |Common Name=&&4-epi Clavulone II&&(4S,12S) - Bis(acetyloxy)-9-oxoprosta- (5Z,7Z,10Z,13Z) -tetrene-1-oic acid methyl ester&& | ||
|Optical=[ alpha ]_D -10.0°(C 0.06, CHCl_3 )[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | |Optical=[ alpha ]_D -10.0°(C 0.06, CHCl_3 )[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | ||
|Mass Spectra=EIMS m/z 446 (M^+ ). HREIMS m/z 446.2328 for C_{25}H_{34}O_7 (M^+ ), calcd 446.2305.[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | |Mass Spectra=EIMS m/z 446 (M^+ ). HREIMS m/z 446.2328 for C_{25}H_{34}O_7 (M^+ ), calcd 446.2305.[[Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884|{{RelationTable/GetFirstAuthor|Reference:Iwashima_M:Okamoto_K:Miyai_Y:Iguchi_K:,Chem. Pharm. Bull. (Tokyo),1999,47,884}}]] | ||
Revision as of 04:19, 24 June 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8043 |
| LipidMaps | LMFA03120024 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV04 |
| 4-epi Clavulone II | |
|---|---|
| |
| Structural Information | |
| (4S,12S) - Bis(acetyloxy)-9-oxoprosta- (5-cis,7-cis,10-cis,13-cis) -tetrene-1-oic acid methyl ester | |
| |
| Formula | C25H34O7 |
| Exact Mass | 446.230453442 |
| Average Mass | 446.53326000000004 |
| SMILES | O(C(C)=O)[C@@](C1=CC=C[C@H](CCC(OC)=O)OC(C)=O)(CC=CCCCCC)C=CC1=O |
| Physicochemical Information | |
| [ α ]D -10.0°(C 0.06, CHCl3) IwashimaMet al. | |
| 4-Epiclavulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iwashima_M et al. | |
| 4-Epiclavulone III was synthesized from clavulone III. Iwashima_M et al. | |
| Spectral Information | |
| Mass Spectra | EIMS m/z 446 (M+). HREIMS m/z 446.2328 for C25}H_{34O7 (M+), calcd 446.2305. IwashimaMet al. |
| UV Spectra | λ EtOH max 229 nm(log ε 4.23),295 nm(log ε 4.14) IwashimaMet al. |
| IR Spectra | ν film max 1738,1698 and 1229cm-1 IwashimaMet al. |
| NMR Spectra | 1H-NMR(500MHz,CDCl3) δ ppm0.88(3H,t,J=7.1Hz),1.21-1.34(6H,m),1.94(2H,q,J=7.3Hz),1.99-2.07(2H,m),2.03(3H,s),2.09(3H,s),2.40(2H,t,J=7.4Hz),2.63(1H,dd,J=7.3,14.2Hz),2.87(1H,dd,J=8.1,14.2Hz),3.68(3H,s),5.21(1H,ddd,J=7.3,8.1,10.9Hz),5.45(1H,dd,J=5.4,5.8Hz),5.53(1H,dt,J=7.3,10.9Hz),6.01(1H,dd,J=5.4,15.6Hz),6.35(1H,d,J=6.1Hz),6.51(1H,d,J=11.3Hz),7.51(1H,d,J=6.1Hz),7.73(1H,ddd,J=1.3,11.3,15.6Hz). IwashimaMet al. 13C-NMR(125MHz,CDCl3) δ ppm14.0,21.0,21.7,22.5,27.4,29.0,29.2,29.7,31.5,35.6,51.7,72.4,85.2,121.3,126.3,133.4,134.7,135.6,136.7,141.0,156.1,169.7,170.2,173.2,194.1. IwashimaMet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|