LBF20406CV09
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR8005 |
| LipidMaps | LMFA03120005 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406CV09 |
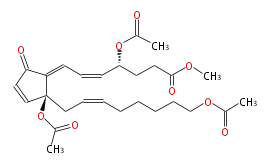
| 20-Acetoxyclavulone I | |
|---|---|
| |
| Structural Information | |
| Methyl-4R- (5-cis,7-trans) -4-acetoxy-7- [ 2S-acetoxy-2- [cis-7-acetoxy-2-octenyl] -5-oxo-3-cyclopentenylidene ] -5-heptenoic acid | |
| |
| Formula | C27H36O9 |
| Exact Mass | 504.23593274999996 |
| Average Mass | 504.56934 |
| SMILES | C(C[C@](OC(C)=O)(C=1)C(=CC=C[C@@H](CCC(=O)OC)OC(C)=O)C(=O)C1)=CCCCCCOC(C)=O |
| Physicochemical Information | |
| [ α ]D -31.1°(C 0.09, CHCl3) IguchiKet al. | |
| 20-Acetoxyclavulones are soluble in MeOH, EtOH, CHCl3, or hexane. | |
| 20-Acetoxyclavulones were isolated from Japanese soft coral, Stolonifer Clavularia viridis Quoy and Gaimard. Iguchi_K et al. | |
| 20-Acetoxyclavulones showed a significant anti-inflammatory effect at 30 mu g/ml by the fertile egg test. Kikuchi_H et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max(EtOH) 230 nm( ε 10900),288 nm( ε 13700) IguchiKet al. |
| IR Spectra | ν max(film)1735,1705,1640,and 1235cm-1 IguchiKet al. |
| NMR Spectra | 1H-NMR(270MHz,CDCl3) δ ppm2.03(3H,s),2.05(6H,s),2.38(2H,t,J=7.5Hz),2.66(1H,dd,J=8,14.5Hz),3.00(1H,dd,J=7,14.5Hz),3.70(3H,s),4.04(2H,t,J=6.6Hz),5.22(1H,m),5.47(1H,m),5.79(1H,m),5.79(1H,m),5.84(1H,t,J=10.2Hz),6.42(1H,d,J=6.3Hz),6.58(1H,dd,J=10.2,12.5Hz),7.27(1H,d,J=12.5Hz), 7.47(1H,d,J=6.3Hz). IguchiKet al.13C-NMR(67.8MHz,CDCl3) δ ppm21.0(q,2C),21.3(q),25.6(t),27.3(t),28.5(t),29.0(t),29.8(t,2C),35.8(t),51.8(q),64.5(t),69.4(d),85.1(s),121.5(d),124.2(d),124.6(d),134.5(d),135.2(d),137.4(s),138.9(d),157.8(d),169.1(s),169.9(s),171.2(s),172.9(s),193.1(s). IguchiKet al. |
| Other Spectra | CD λ ext(EtOH)( Δ ε )nm 247(+2.6),290(-3.6). KikuchiHet al. IguchiKet al. |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|