LBF20406LT02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|SysName=5 (S) ,12 (R) -Dihydroxyeicosa-6 (Z) ,8 (E) ,10 (E) ,14 (Z) -tetraenoic acid | |SysName=5 (S) ,12 (R) -Dihydroxyeicosa-6 (Z) ,8 (E) ,10 (E) ,14 (Z) -tetraenoic acid | ||
|Common Name=&&LEUKOTRIENE B4&& | |Common Name=&&LEUKOTRIENE B4&& | ||
|Solubility=METHANOL | |Solubility=METHANOL [[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] | ||
|Mass Spectra=m/e 336, 319, 301 | |Mass Spectra=m/e 336, 319, 301 [[Reference:Yergey_JA:Kim_HY:Salem_N_Jr:,Anal. Chem.,1986,58,1344|{{RelationTable/GetFirstAuthor|Reference:Yergey_JA:Kim_HY:Salem_N_Jr:,Anal. Chem.,1986,58,1344}}]] | ||
|UV Spectra=METHANOL : 260(<FONT FACE="Symbol">e</FONT> 38,000), 270.5(<FONT FACE="Symbol">e</FONT> 50,000), 281(<FONT FACE="Symbol">e</FONT> 39,000)nm | |UV Spectra=METHANOL : 260(<FONT FACE="Symbol">e</FONT> 38,000), 270.5(<FONT FACE="Symbol">e</FONT> 50,000), 281(<FONT FACE="Symbol">e</FONT> 39,000)nm [[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, D<SUB><FONT SIZE=-1>2</FONT></SUB>O) : <FONT FACE="Symbol">d</FONT> 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, D<SUB><FONT SIZE=-1>2</FONT></SUB>O) : <FONT FACE="Symbol">d</FONT> 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) [[Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409|{{RelationTable/GetFirstAuthor|Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR3101 |
| LipidMaps | LMFA03020001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT02 |
| LEUKOTRIENE B4 | |
|---|---|
| |
| Structural Information | |
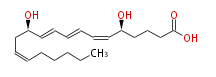
| 5 (S) ,12 (R) -Dihydroxyeicosa-6 (Z) ,8 (E) ,10 (E) ,14 (Z) -tetraenoic acid | |
| |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC=CC[C@H](C=CC=CC=C[C@H](CCCC(O)=O)O)O)CCC |
| Physicochemical Information | |
| METHANOL Corey_EJ et al. | |
| Spectral Information | |
| Mass Spectra | m/e 336, 319, 301 Yergey_JA et al. |
| UV Spectra | METHANOL : 260(e 38,000), 270.5(e 50,000), 281(e 39,000)nm Corey_EJ et al. |
| IR Spectra | |
| NMR Spectra | 1H-NMR(250MHz, D2O) : d 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) Merrer_YL et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|