LBF20406LT02: Difference between revisions
No edit summary |
No edit summary |
||
| (16 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
{{Metabolite | {{Metabolite | ||
|LipidBank=XPR3101 | |LipidBank=XPR3101 | ||
|LipidMaps=LMFA03020001 | |LipidMaps=LMFA03020001 | ||
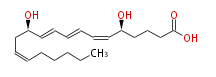
|SysName= | |SysName=(5S,12R) -Dihydroxy- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenoic acid | ||
|Common Name=&& | |Common Name=&&Leukotriene B_4&&(5S,12R) -Dihydroxy- (6Z,8E,10E,14Z) -eicosatraenoic acid&&5 (S) ,12 (R) -Dihydroxyeicosa-6 (Z) ,8 (E) ,10 (E) ,14 (Z) -tetraenoic acid&& | ||
|Solubility=METHANOL [[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] | |Solubility=METHANOL [[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] | ||
|Mass Spectra=m/e 336, 319, 301 [[Reference:Yergey_JA:Kim_HY:Salem_N_Jr:,Anal. Chem.,1986,58,1344|{{RelationTable/GetFirstAuthor|Reference:Yergey_JA:Kim_HY:Salem_N_Jr:,Anal. Chem.,1986,58,1344}}]] | |Mass Spectra=m/e 336, 319, 301 [[Reference:Yergey_JA:Kim_HY:Salem_N_Jr:,Anal. Chem.,1986,58,1344|{{RelationTable/GetFirstAuthor|Reference:Yergey_JA:Kim_HY:Salem_N_Jr:,Anal. Chem.,1986,58,1344}}]] | ||
|UV Spectra=METHANOL : 260( | |UV Spectra=METHANOL : 260( epsilon 38,000), 270.5( epsilon 50,000), 281( epsilon 39,000)nm [[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR(250MHz, D_2 O) : delta 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) [[Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409|{{RelationTable/GetFirstAuthor|Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409}}]] | ||
|Source=Leukotriene B4 is produced by polymorphonuclear leukocytes and macrophages of various animal species upon various stimulations on the cells [[Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1|{{RelationTable/GetFirstAuthor|Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1}}]][[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]]. | |||
|Chemical Synthesis=[[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] {{Image200|LBF20406LT02FT0001.gif}} | |||
|Metabolism=Arachidonic acid is metabolized to leukotrienen A4 with 5,6-epoxide by 5-lipoxygenases, and the product is further transformed to leukotrienee B4 by leukotriene A hydrolase [[Reference:Ford-Hutchinson_AW:Gresser_M:Young_RN:,Annu. Rev. Biochem.,1994,63,383|{{RelationTable/GetFirstAuthor|Reference:Ford-Hutchinson_AW:Gresser_M:Young_RN:,Annu. Rev. Biochem.,1994,63,383}}]]. Leukotriene B4 is metabolized to lose its bioactivities either by omega -oxidation [[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]] or by leukotriene B4 12-hydroxydehydrogenase [[Reference:Yokomizo_T:Izumi_T:Takahashi_T:Kasama_T:Kobayashi_Y:Sato_F:Taketani_Y:Shimizu_T:,J. Biol. Chem.,1993,268,18128|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Izumi_T:Takahashi_T:Kasama_T:Kobayashi_Y:Sato_F:Taketani_Y:Shimizu_T:,J. Biol. Chem.,1993,268,18128}}]]. | |||
|Symbol=LTB4 | |||
|Biological Activity=Leukotriene B4 causes adhesion of leukocytes to endothelial cells, stimulates chemotaxis and chemokinesis of leukocytes[[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]], and enhances superoxide anion production by human polymorphonuclear leukocytes [[Reference:Sumimoto_H:Takeshige_K:Minakami_S:,Biochim. Biophys. Acta,1984,803,271|{{RelationTable/GetFirstAuthor|Reference:Sumimoto_H:Takeshige_K:Minakami_S:,Biochim. Biophys. Acta,1984,803,271}}]]. Leukotriene B4 binds to a specific receptor with 7 transmembrane domains coupled to Gi/Go or Gq protein [[Reference:Yokomizo_T:Izumi_T:Chang_K:Takuwa_Y:Shimizu_T:,Nature,1997,387,620|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Izumi_T:Chang_K:Takuwa_Y:Shimizu_T:,Nature,1997,387,620}}]]. | |||
|Genetic Information=cDNA and genomic DNA of 5-lipoxygenase [[Reference:Funk_CD:,Prog Nucleic Acid Res Mol Biol.,1993,45,67|{{RelationTable/GetFirstAuthor|Reference:Funk_CD:,Prog Nucleic Acid Res Mol Biol.,1993,45,67}}]] and cDNA for Leukotriene A hydrolase [[Reference:Funk_CD:,Prog Nucleic Acid Res Mol Biol.,1993,45,67|{{RelationTable/GetFirstAuthor|Reference:Funk_CD:,Prog Nucleic Acid Res Mol Biol.,1993,45,67}}]] were cloned. cDNA for leukotriene B 12-hydroxydehydrogenase was cloned [[Reference:Yokomizo_T:Ogawa_Y:Uozumi_N:Kume_K:Izumi_T:Shimizu_T:,J. Biol. Chem.,1996,271,2844|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Ogawa_Y:Uozumi_N:Kume_K:Izumi_T:Shimizu_T:,J. Biol. Chem.,1996,271,2844}}]]. | |||
}} | |||
{{MassbankSpectra| | |||
UT000280 | |||
UT000281 | |||
UT000282 | |||
UT000283 | |||
UT000284 | |||
UT000285 | |||
UT000286 | |||
UT000287 | |||
UT000288 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 00:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3101 |
| LipidMaps | LMFA03020001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT02 |
| Leukotriene B4 | |
|---|---|
| |
| Structural Information | |
| (5S,12R) -Dihydroxy- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenoic acid | |
| |
| LTB4 | |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC=CC[C@H](C=CC=CC=C[C@H](CCCC(O)=O)O)O)CCC |
| Physicochemical Information | |
| METHANOL Corey_EJ et al. | |
| Leukotriene B4 is produced by polymorphonuclear leukocytes and macrophages of various animal species upon various stimulations on the cells Samuelsson_B et al. Hammarstrom_S . | |
Corey_EJ et al. 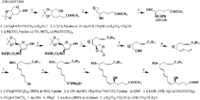 | |
| Arachidonic acid is metabolized to leukotrienen A4 with 5,6-epoxide by 5-lipoxygenases, and the product is further transformed to leukotrienee B4 by leukotriene A hydrolase Ford-Hutchinson_AW et al.. Leukotriene B4 is metabolized to lose its bioactivities either by omega -oxidation Hammarstrom_S or by leukotriene B4 12-hydroxydehydrogenase Yokomizo_T et al.. | |
| Leukotriene B4 causes adhesion of leukocytes to endothelial cells, stimulates chemotaxis and chemokinesis of leukocytes Hammarstrom_S , and enhances superoxide anion production by human polymorphonuclear leukocytes Sumimoto_H et al.. Leukotriene B4 binds to a specific receptor with 7 transmembrane domains coupled to Gi/Go or Gq protein Yokomizo_T et al.. | |
| cDNA and genomic DNA of 5-lipoxygenase Funk_CD and cDNA for Leukotriene A hydrolase Funk_CD were cloned. cDNA for leukotriene B 12-hydroxydehydrogenase was cloned Yokomizo_T et al.. | |
| Spectral Information | |
| Mass Spectra | m/e 336, 319, 301 Yergey_JA et al. |
| UV Spectra | METHANOL : 260( ε 38,000), 270.5( ε 50,000), 281( ε 39,000)nm Corey_EJ et al. |
| IR Spectra | |
| NMR Spectra | 1H-NMR(250MHz, D2O) : δ 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) Merrer_YL et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|








