LBF20406LT04: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR3113 | |LipidBank=XPR3113 | ||
|LipidMaps=LMFA03020011 | |LipidMaps=LMFA03020011 | ||
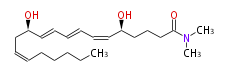
|SysName=N,N- | |SysName=N,N-Dimethyl- (5S,12R) -dihydroxy- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenamide | ||
|Common Name=&& | |Common Name=&&Leukotriene B_4 dimethylamide&&N,N-Dimethyl- (5S,12R) -dihydroxy- (6Z,8E,10E,14Z) -eicosatetraenamide&& | ||
|Source= | |Source= | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Latest revision as of 06:23, 9 November 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3113 |
| LipidMaps | LMFA03020011 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT04 |
| Leukotriene B4dimethylamide | |
|---|---|
| |
| Structural Information | |
| N,N-Dimethyl- (5S,12R) -dihydroxy- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenamide | |
| |
| Formula | C22H37NO3 |
| Exact Mass | 363.27734405499996 |
| Average Mass | 363.53412000000003 |
| SMILES | C(=CC[C@@H](O)C=CC=CC=C[C@H](CCCC(N(C)C)=O)O)CCCCC |
| Physicochemical Information | |
| Leukotriene B4 dimethylamide inhibits LTB4 induced degranulation of human neutrophils at the concentration of 130 nM (Ki) and release of lysozyme of PMNL. Falcone_RC et al. Shimazaki_T et al. Showell_HJ et al. The effect may be due to the antagonistic effect against LTB4 receptor. Falcone_RC et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|