LBF20406LT09: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=XPR3118 | |LipidBank=XPR3118 | ||
|LipidMaps=LMFA03020016 | |LipidMaps=LMFA03020016 | ||
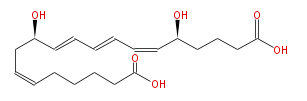
|SysName=(5S,12R) -Dihydroxy- (6-cis,8-trans,10-trans,14-cis) - | |SysName=(5S,12R) -Dihydroxy- (6-cis,8-trans,10-trans,14-cis) -eicosatetraenoic acid | ||
|Common Name=&&20-carboxy Leukotriene B_4&&(5S,12R) -Dihydroxy- (6Z,8E,10E,14Z) -eicosatetraenoic acid | |Common Name=&&20-carboxy Leukotriene B_4&&(5S,12R) -Dihydroxy- (6Z,8E,10E,14Z) -eicosatetraenoic acid | ||
|Source=20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils | |Source=20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils | ||
Revision as of 07:45, 2 June 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3118 |
| LipidMaps | LMFA03020016 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT09 |
| 20-carboxy Leukotriene B4 | |
|---|---|
| |
| Structural Information | |
| (5S,12R) -Dihydroxy- (6-cis,8-trans,10-trans,14-cis) -eicosatetraenoic acid | |
| |
| Formula | C20H30O6 |
| Exact Mass | 366.204238692 |
| Average Mass | 366.4486 |
| SMILES | C(CCCC(O)=O)C=CC[C@H](C=CC=CC=C[C@H](CCCC(O)=O)O)O |
| Physicochemical Information | |
| 20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils | |
| The biological activity of 20-carboxy LTB4 is only about 2.6% compared to that of LTB4 in causing PMNL degradation. Feinmark_SJ et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|