LBF20406LT11: Difference between revisions
No edit summary |
No edit summary |
||
| (17 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR3120 | |LipidBank=XPR3120 | ||
|LipidMaps=LMFA03020018 | |LipidMaps=LMFA03020018 | ||
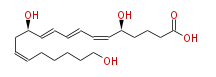
|SysName=5S,12R,20- | |SysName=(5S,12R,20) -Trihydroxy- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenoic acid | ||
|Common Name=&&20-hydroxy Leukotriene | |Common Name=&&20-hydroxy Leukotriene B_4&&(5S,12R,20) -Trihydroxy- (6Z,8E,10E,14Z) -eicosatetraenoic acid&&20-hydroxy LTB4&& | ||
|Source= | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
|Biological Activity=20-hydroxy LTB4 is commonly accepted as a inactive metabolite of LTB4 but reported to contract parenchymal strips from guinea pig lung [[Reference:Hansson_G:Lindgren_JA:Dahlen_SE:Hedqvist_P:Samuelsson_B:,FEBS Lett.,1981,130,107|{{RelationTable/GetFirstAuthor|Reference:Hansson_G:Lindgren_JA:Dahlen_SE:Hedqvist_P:Samuelsson_B:,FEBS Lett.,1981,130,107}}]] and as a good ligand form BLT2 receptor.[[Reference:Yokomizo_T:Kato_K:Hagiya_H:Izumi_T:Shimizu_T:,J. Biol. Chem.,2001,276,12454|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Kato_K:Hagiya_H:Izumi_T:Shimizu_T:,J. Biol. Chem.,2001,276,12454}}]] | |||
}} | |||
{{MassbankSpectra| | |||
UT000289 | |||
UT000290 | |||
UT000291 | |||
UT000292 | |||
UT000293 | |||
UT000294 | |||
UT000295 | |||
UT000296 | |||
UT000297 | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 08:51, 5 April 2016
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3120 |
| LipidMaps | LMFA03020018 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT11 |
| 20-hydroxy Leukotriene B4 | |
|---|---|
| |
| Structural Information | |
| (5S,12R,20) -Trihydroxy- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenoic acid | |
| |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CCC=CC[C@H](C=CC=CC=C[C@H](CCCC(O)=O)O)O)CCO |
| Physicochemical Information | |
| 20-hydroxy LTB4 is commonly accepted as a inactive metabolite of LTB4 but reported to contract parenchymal strips from guinea pig lung Hansson_G et al. and as a good ligand form BLT2 receptor. Yokomizo_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|








