LBF20406LT13: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|SysName=5 (S) -Hydroxy-6 (R) -S-cysteinylglycinyleicosa-7 (E) ,9 (E) ,11 (Z) ,14 (Z) -tetraenoic acid | |SysName=5 (S) -Hydroxy-6 (R) -S-cysteinylglycinyleicosa-7 (E) ,9 (E) ,11 (Z) ,14 (Z) -tetraenoic acid | ||
|Common Name=&&LEUKOTRIENE D4&& | |Common Name=&&LEUKOTRIENE D4&& | ||
|Solubility=METHANOL | |Solubility=METHANOL [[Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601|{{RelationTable/GetFirstAuthor|Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601}}]] | ||
|Mass Spectra=N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 | |Mass Spectra=N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 [[Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601|{{RelationTable/GetFirstAuthor|Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601}}]] | ||
|UV Spectra=<FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>M</FONT></SUP><SUP><FONT SIZE=-1>e</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 270(<FONT FACE="Symbol">e</FONT> 32,000), 280(<FONT FACE="Symbol">e</FONT> 40,000), 290(<FONT FACE="Symbol">e</FONT> 31,000)nm | |UV Spectra=<FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>M</FONT></SUP><SUP><FONT SIZE=-1>e</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 270(<FONT FACE="Symbol">e</FONT> 32,000), 280(<FONT FACE="Symbol">e</FONT> 40,000), 290(<FONT FACE="Symbol">e</FONT> 31,000)nm [[Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710|{{RelationTable/GetFirstAuthor|Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR3301 |
| LipidMaps | LMFA03020006 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT13 |
| LEUKOTRIENE D4 | |
|---|---|
| |
| Structural Information | |
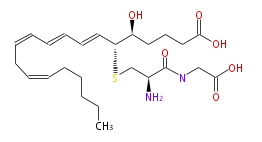
| 5 (S) -Hydroxy-6 (R) -S-cysteinylglycinyleicosa-7 (E) ,9 (E) ,11 (Z) ,14 (Z) -tetraenoic acid | |
| |
| Formula | C25H40N2O6S |
| Exact Mass | 496.260707712 |
| Average Mass | 496.66098 |
| SMILES | C(=CC=CC=C[C@@H](SC[C@@H](C(=O)NCC(O)=O)N)[C@H](CCCC(O)=O)O)CC=CCCCCC |
| Physicochemical Information | |
| METHANOL Morris_HR et al. | |
| Spectral Information | |
| Mass Spectra | N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M+), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 Morris_HR et al. |
| UV Spectra | l MeOHmax = 270(e 32,000), 280(e 40,000), 290(e 31,000)nm Lewis_RA et al. |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|