LBF20406LT13: Difference between revisions
No edit summary |
No edit summary |
||
| Line 11: | Line 11: | ||
|Mass Spectra=N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 [[Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601|{{RelationTable/GetFirstAuthor|Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601}}]] | |Mass Spectra=N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 [[Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601|{{RelationTable/GetFirstAuthor|Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601}}]] | ||
|UV Spectra=<FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>M</FONT></SUP><SUP><FONT SIZE=-1>e</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 270(<FONT FACE="Symbol">e</FONT> 32,000), 280(<FONT FACE="Symbol">e</FONT> 40,000), 290(<FONT FACE="Symbol">e</FONT> 31,000)nm [[Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710|{{RelationTable/GetFirstAuthor|Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710}}]] | |UV Spectra=<FONT FACE="Symbol">l</FONT> <SUP><FONT SIZE=-1>M</FONT></SUP><SUP><FONT SIZE=-1>e</FONT></SUP><SUP><FONT SIZE=-1>O</FONT></SUP><SUP><FONT SIZE=-1>H</FONT></SUP><SUB><FONT SIZE=-1>m</FONT></SUB><SUB><FONT SIZE=-1>a</FONT></SUB><SUB><FONT SIZE=-1>x</FONT></SUB> = 270(<FONT FACE="Symbol">e</FONT> 32,000), 280(<FONT FACE="Symbol">e</FONT> 40,000), 290(<FONT FACE="Symbol">e</FONT> 31,000)nm [[Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710|{{RelationTable/GetFirstAuthor|Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710}}]] | ||
|Source=Leukotriene D4 is produced by basophils, eosinophils and macrophages of various animal species upon various stimulations on the cells [[Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1|{{RelationTable/GetFirstAuthor|Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1}}]][[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]];>. | |||
|Chemical Synthesis=[[Reference:Cohen_N:Banner_BL:Lopresti_RJ:Wong_F:Rosenberger_M:Liu_YY:Thom_E:Liebman_AA:,J. Am. Chem. Soc.,1983,105,3661|{{RelationTable/GetFirstAuthor|Reference:Cohen_N:Banner_BL:Lopresti_RJ:Wong_F:Rosenberger_M:Liu_YY:Thom_E:Liebman_AA:,J. Am. Chem. Soc.,1983,105,3661}}]];> {{Image200|XPR3301FT0001.gif}} | |||
|Metabolism= <FONT FACE="Symbol">g</FONT>-Glutamyl transpeptidase hydrolyzes the glutathione moiety of leukotriene C4 and produces leukotriene D4 liberating glutamic acid [[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]];>, | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 22:00, 24 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3301 |
| LipidMaps | LMFA03020006 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT13 |
| LEUKOTRIENE D4 | |
|---|---|
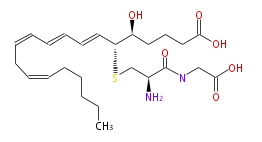
| |
| Structural Information | |
| 5 (S) -Hydroxy-6 (R) -S-cysteinylglycinyleicosa-7 (E) ,9 (E) ,11 (Z) ,14 (Z) -tetraenoic acid | |
| |
| Formula | C25H40N2O6S |
| Exact Mass | 496.260707712 |
| Average Mass | 496.66098 |
| SMILES | C(=CC=CC=C[C@@H](SC[C@@H](C(=O)NCC(O)=O)N)[C@H](CCCC(O)=O)O)CC=CCCCCC |
| Physicochemical Information | |
| METHANOL Morris_HR et al. | |
| Leukotriene D4 is produced by basophils, eosinophils and macrophages of various animal species upon various stimulations on the cells Samuelsson_B et al. Hammarstrom_S ;>. | |
|
Cohen_N et al.;> File:XPR3301FT0001.gif | |
| g-Glutamyl transpeptidase hydrolyzes the glutathione moiety of leukotriene C4 and produces leukotriene D4 liberating glutamic acid Hammarstrom_S ;>, | |
| Spectral Information | |
| Mass Spectra | N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M+), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 Morris_HR et al. |
| UV Spectra | l MeOHmax = 270(e 32,000), 280(e 40,000), 290(e 31,000)nm Lewis_RA et al. |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|