LBF20406LT13: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=XPR3301 | |LipidBank=XPR3301 | ||
|LipidMaps=LMFA03020006 | |LipidMaps=LMFA03020006 | ||
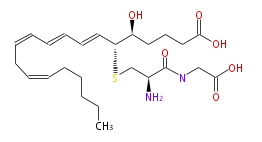
|SysName= | |SysName=5S-Hydroxy-6R-S-cysteinylglycinyl- (trans-7,trans-9,cis-11,cis-14) -eicosatetraenoic acid | ||
|Common Name=&& | |Common Name=&&Leukotriene D_4&&5S-Hydroxy-6R-S-cysteinylglycinyl- (7E,9E,11Z,14Z) -eicosatetraenoic acid&&5 (S) -Hydroxy-6 (R) -S-cysteinylglycinyleicosa-7 (E) ,9 (E) ,11 (Z) ,14 (Z) -tetraenoic acid&& | ||
|Solubility=METHANOL [[Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601|{{RelationTable/GetFirstAuthor|Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601}}]] | |Solubility=METHANOL [[Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601|{{RelationTable/GetFirstAuthor|Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601}}]] | ||
|Mass Spectra=N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M | |Mass Spectra=N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M^+ ), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 [[Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601|{{RelationTable/GetFirstAuthor|Reference:Morris_HR:Taylor_GW:Rokach_J:Girard_Y:Piper_PJ:Tippins_JR:Samhoun_MN:,Prostaglandins,1980,20,601}}]] | ||
|UV Spectra= | |UV Spectra= lambda ^{MeOH}_{max} = 270( epsilon 32,000), 280( epsilon 40,000), 290( epsilon 31,000)nm [[Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710|{{RelationTable/GetFirstAuthor|Reference:Lewis_RA:Austen_KF:Drazen_JM:Clark_DA:Marfat_A:Corey_EJ:,Proc. Natl. Acad. Sci. U. S. A.,1980,77,3710}}]] | ||
|Source=Leukotriene D4 is produced by basophils, eosinophils and macrophages of various animal species upon various stimulations on the cells [[Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1|{{RelationTable/GetFirstAuthor|Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1}}]][[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]]. | |Source=Leukotriene D4 is produced by basophils, eosinophils and macrophages of various animal species upon various stimulations on the cells [[Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1|{{RelationTable/GetFirstAuthor|Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1}}]][[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]]. | ||
|Chemical Synthesis=[[Reference:Cohen_N:Banner_BL:Lopresti_RJ:Wong_F:Rosenberger_M:Liu_YY:Thom_E:Liebman_AA:,J. Am. Chem. Soc.,1983,105,3661|{{RelationTable/GetFirstAuthor|Reference:Cohen_N:Banner_BL:Lopresti_RJ:Wong_F:Rosenberger_M:Liu_YY:Thom_E:Liebman_AA:,J. Am. Chem. Soc.,1983,105,3661}}]] {{Image200|LBF20406LT13FT0001.gif}} | |Chemical Synthesis=[[Reference:Cohen_N:Banner_BL:Lopresti_RJ:Wong_F:Rosenberger_M:Liu_YY:Thom_E:Liebman_AA:,J. Am. Chem. Soc.,1983,105,3661|{{RelationTable/GetFirstAuthor|Reference:Cohen_N:Banner_BL:Lopresti_RJ:Wong_F:Rosenberger_M:Liu_YY:Thom_E:Liebman_AA:,J. Am. Chem. Soc.,1983,105,3661}}]] {{Image200|LBF20406LT13FT0001.gif}} | ||
|Metabolism= | |Metabolism= gamma -Glutamyl transpeptidase hydrolyzes the glutathione moiety of leukotriene C4 and produces leukotriene D4 liberating glutamic acid [[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]], | ||
|Symbol=LTD4 | |||
|Biological Activity=Leukotriene D4 stimulates airway smooth muscles and causes bronchoconstriction. Vascular permeability is enhanced. Gasstrointestinal smooth muscles are contracted [[Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1|{{RelationTable/GetFirstAuthor|Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1}}]][[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]]. Leukotriene D4 binds to a receptor with 7 transmembrane domains coupled to Gi alpha /o protein (CysLT1) with an affinity higher by two orders of magnitude than that of leukotriene C4 [[Reference:Lynch_KR:ONeill_GP:Liu_Q:Im_DS:Sawyer_N:Metters_KM:Coulombe_N:Abramovitz_M:Figueroa_DJ:Zeng_Z_et_al:,Nature,1999,399,789|{{RelationTable/GetFirstAuthor|Reference:Lynch_KR:ONeill_GP:Liu_Q:Im_DS:Sawyer_N:Metters_KM:Coulombe_N:Abramovitz_M:Figueroa_DJ:Zeng_Z_et_al:,Nature,1999,399,789}}]]. | |||
|Genetic Information=cDNA for CysLT1 was cloned [[Reference:Lynch_KR:ONeill_GP:Liu_Q:Im_DS:Sawyer_N:Metters_KM:Coulombe_N:Abramovitz_M:Figueroa_DJ:Zeng_Z_et_al:,Nature,1999,399,789|{{RelationTable/GetFirstAuthor|Reference:Lynch_KR:ONeill_GP:Liu_Q:Im_DS:Sawyer_N:Metters_KM:Coulombe_N:Abramovitz_M:Figueroa_DJ:Zeng_Z_et_al:,Nature,1999,399,789}}]]. | |||
}} | |||
{{MassbankSpectra| | |||
UT000316 | |||
UT000317 | |||
UT000318 | |||
UT000319 | |||
UT000320 | |||
UT000321 | |||
UT000322 | |||
UT000323 | |||
UT000324 | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 00:00, 17 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3301 |
| LipidMaps | LMFA03020006 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT13 |
| Leukotriene D4 | |
|---|---|
| |
| Structural Information | |
| 5S-Hydroxy-6R-S-cysteinylglycinyl- (trans-7,trans-9,cis-11,cis-14) -eicosatetraenoic acid | |
| |
| LTD4 | |
| Formula | C25H40N2O6S |
| Exact Mass | 496.260707712 |
| Average Mass | 496.66098 |
| SMILES | C(=CC=CC=C[C@@H](SC[C@@H](C(=O)NCC(O)=O)N)[C@H](CCCC(O)=O)O)CC=CCCCCC |
| Physicochemical Information | |
| METHANOL Morris_HR et al. | |
| Leukotriene D4 is produced by basophils, eosinophils and macrophages of various animal species upon various stimulations on the cells Samuelsson_B et al. Hammarstrom_S . | |
|
Cohen_N et al. | |
| gamma -Glutamyl transpeptidase hydrolyzes the glutathione moiety of leukotriene C4 and produces leukotriene D4 liberating glutamic acid Hammarstrom_S , | |
| Leukotriene D4 stimulates airway smooth muscles and causes bronchoconstriction. Vascular permeability is enhanced. Gasstrointestinal smooth muscles are contracted Samuelsson_B et al. Hammarstrom_S . Leukotriene D4 binds to a receptor with 7 transmembrane domains coupled to Gi alpha /o protein (CysLT1) with an affinity higher by two orders of magnitude than that of leukotriene C4 Lynch_KR et al.. | |
| cDNA for CysLT1 was cloned Lynch_KR et al.. | |
| Spectral Information | |
| Mass Spectra | N-ACETYL, 5-TRIMETHYLSILYL ETHER DIMETHYL ESTER derivative ; 638(M+), 623, 607, 548, 508, 405, 404, 315, 314, 274, 273 Morris_HR et al. |
| UV Spectra | λ MeOH max = 270( ε 32,000), 280( ε 40,000), 290( ε 31,000)nm Lewis_RA et al. |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|