LBF20406LT14
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3201 |
| LipidMaps | LMFA03020003 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT14 |
| Leukotriene C4 | |
|---|---|
| |
| Structural Information | |
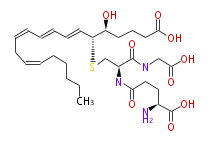
| 5S-Hydroxy-6R-S-γ-glutamylcysteinylglycinyl- (trans-7,trans-9,cis-11,cis-14) -eicosatetraenoic acid | |
| |
| LTC4 | |
| Formula | C30H47N3O9S |
| Exact Mass | 625.303300807 |
| Average Mass | 625.7750000000001 |
| SMILES | C(O)(=O)[C@H](CCC(=O)N[C@@H](C(=O)NCC(O)=O)CS[C@H](C=CC=CC=CCC=CCCCCC)[C@H](O)CCCC(O)=O)N |
| Physicochemical Information | |
| METHANOL Corey_EJ et al. | |
| Leukotriene C4 is produced by polymorphonuclear leukocytes, mast cells and macrophages of various animal species upon various stimulations on the cells Samuelsson_B et al. Hammarstrom_S . | |
Corey_EJ et al.  | |
| Arachidonic acid is metabolized to leukotrienen A4 with 5,6-epoxide by 5-lipoxygenases, and the product is further transformed to leukotrienee C4 incorporating glutathione by the catalysis of leukotriene C synthase Samuelsson_B et al.. | |
| Leukotriene C4 is a potent stimulator of airway smooth muscles and causes bronchoconstriction as demonstrated in vitro and in vivo experiments, and gastrointestinal smooth muscles are also contracted. Vascular permeability is enhanced by leukotriene C4 at concentrations lower by 3-4 orders of magnitude than histamine Hammarstrom_S . Gasstrointestinal smooth muscles are contracted Samuelsson_B et al. Hammarstrom_S . Leukotriene C4 binds to a receptor with 7 transmembrane domains coupled to Gi α /o protein (CysLT1) with an affinity lower by two orders of magnitude than that of leukotriene D4 Lynch_KR et al.. | |
| cDNA and genomic DNA of 5-lipoxygenase Funk_CD and those for leukotriene C synthase Lam_BK et al. Penrose_JF et al. were cloned. cDNA for CysLT1 was cloned Lynch_KR et al.. | |
| Spectral Information | |
| Mass Spectra | the Chart Pace-Asciak_CR |
| UV Spectra | λ MeOH max = 270( ε 32,000), 280( ε 40,000), 290( ε 31,000)nm Corey_EJ et al. |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|