LBF20406LT17
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3401 |
| LipidMaps | LMFA03020002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT17 |
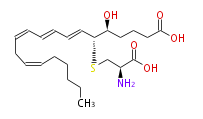
| Leukotriene E4 | |
|---|---|
| |
| Structural Information | |
| 5S-Hydroxy-6R-S-cysteinyl- (trans-7,trans-9,cis-11,cis-14) -eicosatetraenoic acid | |
| |
| LTE4 | |
| Formula | C23H37NO5S |
| Exact Mass | 439.239243989 |
| Average Mass | 439.60962000000006 |
| SMILES | OC(=O)CCC[C@@H]([C@@H](C=CC=CC=CCC=CCCCCC)SC[C@H](N)C(O)=O)O |
| Physicochemical Information | |
| DIMETHYL ESTER ; [ α ]20 D =+35.2° RosenbergerMet al. | |
| METHANOL RosenbergerMet al. CohenNet al. | |
| When leukotriene C4 was injected into male subjects, leukotriene E4 was found as a major urinary metabolite Orning_L et al.. Incubation of leukotriene D4 with human polymorphonuclear leukocytes produced leukotriene E4 Lee_CW et al.. | |
Cohen_N et al.  | |
| Leukotriene D4 is converted to E4 by extracellular action of a dipeptidase released from granules of human polymorphonuclear leukocytes Lee_CW et al.. Leukotriene E4 is transformed to leukotriene F4 by gamma -glultamyltransferase in the presence of glutathione, and to N-acetyl leukotriene E4 by incubation with rat liver microsomes Hammarstrom_S et al.. | |
| Leukotriene E4 stimulates airway smooth muscles from different animal species, and is less potent than C4 in contracting isolated guinea pig ileum Hammarstrom_S . | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | MONO-POTASSIUM SALT; λ = 270( ε 40000), 280( ε 49400), 291nm( ε 40000) CohenNet al. |
| IR Spectra | |
| NMR Spectra | DIMETHYL ESTER ; 1H-NMR(CDCl3) : δ 6.33(dd, J=14.5Hz, 10Hz, 1H, 10CH), 6.0(t, J=10Hz, 1H, 11-CH), 5.62(dd, J=14.4, 9.6Hz, 1H, 7-CH), 5.3(m, J=10,9Hz, 1H, 14-CH), 3.71 and 3.62(2S, 6H, OCH3), 3.65(m, 1H, 5-CH), 3.4(m, 1H, 6-CH), 2.95(t, J=9Hz, 2H, 13-CH), 2.02(m, 2H, 16-CH), 0.86(t, J=6Hz, 3H, 20-CH) RosenbergerMet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|