LBF20407HO05: Difference between revisions
No edit summary |
No edit summary |
||
| (16 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=DFA8139 | |LipidBank=DFA8139 | ||
|LipidMaps=LMFA03060030 | |LipidMaps=LMFA03060030 | ||
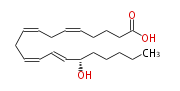
|SysName=15R- | |SysName=15R-Hydroxy- (cis-5,cis-8,trans-10,cis-14) -eicosatetraenoic acid | ||
|UV Spectra= | |Common Name=&&15R-Hydroxy- (5Z,8Z,10E,14Z) -eicosatetraenoic acid&& | ||
|UV Spectra= lambda max: 236nm epsilon : 27,000 | |||
|Source= | |||
|Chemical Synthesis= | |||
|Metabolism=(±)15-HETE, an autoxidation product of arachidonic acid, is comprised of an equal mixture of 15(R)- and 15(S)-HETE. Aspirin-inactivated human recombinant and ovine COX-2 metabolizes arachidonic acid to 15(R)-HETE [[Reference:Lecomte_M:Laneuville_O:Ji_C:DeWitt_DL:Smith_WL:,J. Biol. Chem.,1994,269,13207|{{RelationTable/GetFirstAuthor|Reference:Lecomte_M:Laneuville_O:Ji_C:DeWitt_DL:Smith_WL:,J. Biol. Chem.,1994,269,13207}}]][[Reference:Capdevila_JH:Morrow_JD:Belosludtsev_YY:Beauchamp_DR:DuBois_RN:Falck_JR:,Biochemistry,1995,34,3325|{{RelationTable/GetFirstAuthor|Reference:Capdevila_JH:Morrow_JD:Belosludtsev_YY:Beauchamp_DR:DuBois_RN:Falck_JR:,Biochemistry,1995,34,3325}}]]. | |||
|Symbol=15(R)-HETE | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 07:34, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8139 |
| LipidMaps | LMFA03060030 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20407HO05 |
| 15R-Hydroxy- (5Z,8Z,10E,14Z) -eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 15R-Hydroxy- (cis-5,cis-8,trans-10,cis-14) -eicosatetraenoic acid | |
| |
| 15(R)-HETE | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC[C@H](C=CC=CCC=CCC=CCCCC(O)=O)O)CC |
| Physicochemical Information | |
| (±)15-HETE, an autoxidation product of arachidonic acid, is comprised of an equal mixture of 15(R)- and 15(S)-HETE. Aspirin-inactivated human recombinant and ovine COX-2 metabolizes arachidonic acid to 15(R)-HETE Lecomte_M et al. Capdevila_JH et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 236nm ε : 27,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|