LBF20407HO07: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=- | |LipidMaps=- | ||
|SysName=15 (S) -Hydroxy-5,8,11,13- (Z,Z,Z,E) -eicosatetraenoic acid | |SysName=15 (S) -Hydroxy-5,8,11,13- (Z,Z,Z,E) -eicosatetraenoic acid | ||
|Solubility=DIETHYL ETHER | |Solubility=DIETHYL ETHER [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | ||
|Mass Spectra=METHYL ESTER TMS ETHER ; m/e 406(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 391, 335, 316, 305, 225, 173 | |Mass Spectra=METHYL ESTER TMS ETHER ; m/e 406(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 391, 335, 316, 305, 225, 173 [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | ||
|UV Spectra=METHYL ESTER ; <FONT FACE="Symbol">l</FONT> (ISOOCTANE) = 236nm(<FONT FACE="Symbol">e</FONT> 27200) | |UV Spectra=METHYL ESTER ; <FONT FACE="Symbol">l</FONT> (ISOOCTANE) = 236nm(<FONT FACE="Symbol">e</FONT> 27200) [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | ||
|NMR Spectra=METHYL ESTER ; <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(90MHz) : <FONT FACE="Symbol">d</FONT> 6.56(dd, J=11, 14.5Hz, 1H, 13-CH), 6.02(dd, J=11,11Hz, 1H, 12-CH), 5.7(dd, J=6.8, 14.5Hz, 1H,14-CH), 5.51(dd, J=6,11Hz, 1H, 11-CH), 4.16(dd, J=6.8, 6.8Hz, 1H, 15-CH), 3.69(s, 3H, OCH<SUB><FONT SIZE=-1>3</FONT></SUB>), 2.98(2H, 10-CH), 2.84(2H, 7-CH), 2.35(2H, 2-CH), 2.08(2H, 4-CH), 1.47(2H, 16-CH), 2.3-0.9(11H) | |NMR Spectra=METHYL ESTER ; <SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(90MHz) : <FONT FACE="Symbol">d</FONT> 6.56(dd, J=11, 14.5Hz, 1H, 13-CH), 6.02(dd, J=11,11Hz, 1H, 12-CH), 5.7(dd, J=6.8, 14.5Hz, 1H,14-CH), 5.51(dd, J=6,11Hz, 1H, 11-CH), 4.16(dd, J=6.8, 6.8Hz, 1H, 15-CH), 3.69(s, 3H, OCH<SUB><FONT SIZE=-1>3</FONT></SUB>), 2.98(2H, 10-CH), 2.84(2H, 7-CH), 2.35(2H, 2-CH), 2.08(2H, 4-CH), 1.47(2H, 16-CH), 2.3-0.9(11H) [[Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115|{{RelationTable/GetFirstAuthor|Reference:Baldwin_JE:Davies_DI:Hughes_L:Gutterridge_NJA:,J. Chem. Soc. Perkin I,1979,,115}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR6103 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20407HO07 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
| |
| Structural Information | |
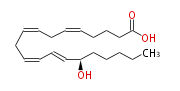
| 15 (S) -Hydroxy-5,8,11,13- (Z,Z,Z,E) -eicosatetraenoic acid | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC[C@@H](C=CC=CCC=CCC=CCCCC(O)=O)O)CC |
| Physicochemical Information | |
| DIETHYL ETHER Baldwin_JE et al. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER TMS ETHER ; m/e 406(M+), 391, 335, 316, 305, 225, 173 Baldwin_JE et al. |
| UV Spectra | METHYL ESTER ; l (ISOOCTANE) = 236nm(e 27200) Baldwin_JE et al. |
| IR Spectra | |
| NMR Spectra | METHYL ESTER ; 1H-NMR(90MHz) : d 6.56(dd, J=11, 14.5Hz, 1H, 13-CH), 6.02(dd, J=11,11Hz, 1H, 12-CH), 5.7(dd, J=6.8, 14.5Hz, 1H,14-CH), 5.51(dd, J=6,11Hz, 1H, 11-CH), 4.16(dd, J=6.8, 6.8Hz, 1H, 15-CH), 3.69(s, 3H, OCH3), 2.98(2H, 10-CH), 2.84(2H, 7-CH), 2.35(2H, 2-CH), 2.08(2H, 4-CH), 1.47(2H, 16-CH), 2.3-0.9(11H) Baldwin_JE et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|