LBF20503HO09: Difference between revisions
No edit summary |
No edit summary |
||
| Line 8: | Line 8: | ||
|SysName=5S-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid | |SysName=5S-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid | ||
|Common Name=&&5S-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid&& | |Common Name=&&5S-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid&& | ||
|UV Spectra= | |UV Spectra= lambda max: 236nm epsilon : 23,000 | ||
|Source=The synthesis of 5(S)-HEPE from EPA by tissue homogenates has been demonstrated [[Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349|{{RelationTable/GetFirstAuthor|Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349}}]][[Reference:Kulkarni_PS:Srinivasan_BD:,Prostaglandins,1986,31,1159|{{RelationTable/GetFirstAuthor|Reference:Kulkarni_PS:Srinivasan_BD:,Prostaglandins,1986,31,1159}}]]. | |Source=The synthesis of 5(S)-HEPE from EPA by tissue homogenates has been demonstrated [[Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349|{{RelationTable/GetFirstAuthor|Reference:Kulkarni_PS:Kaufman_PL:Srinivasan_BD:,J. Ocul. Pharmacol.,1987,3,349}}]][[Reference:Kulkarni_PS:Srinivasan_BD:,Prostaglandins,1986,31,1159|{{RelationTable/GetFirstAuthor|Reference:Kulkarni_PS:Srinivasan_BD:,Prostaglandins,1986,31,1159}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Revision as of 14:00, 19 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8118 |
| LipidMaps | LMFA03070001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20503HO09 |
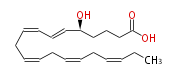
| 5S-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid | |
|---|---|
| |
| Structural Information | |
| 5S-hydroxy-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid | |
| |
| 5(S)-HEPE | |
| Formula | C20H30O3 |
| Exact Mass | 318.21949482599996 |
| Average Mass | 318.4504 |
| SMILES | C(CC=CCC=CCC=CC=CC(CCCC(O)=O)O)=CCC |
| Physicochemical Information | |
| The synthesis of 5(S)-HEPE from EPA by tissue homogenates has been demonstrated Kulkarni_PS et al. Kulkarni_PS et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 236nm ε : 23,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|