LBF20503HP01
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8096 |
| LipidMaps | - |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20503HP01 |
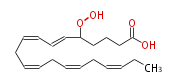
| 5-Hydroperoxy-6,8,11,14,17-eicosapentaenoic acid | |
|---|---|
| |
| Structural Information | |
| 5-Hydroperoxy-6,8,11,14,17-eicosapentaenoic acid | |
| |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC=CCC=CCC=CC=CC(OO)CCCC(O)=O)=CCC |
| Physicochemical Information | |
| Autooxidation of icosapentaenoate Yamauchi_R et al.. Oxidation of icosapentaenoate by singlet-oxygen Yamauchi_R et al.. Oxidation of icosapentaenoate in the presence of mioglobin. It is produced from icosapentaenoate by 5-lipoxygenate of arachidonic acid Lee_TH et al.. | |
| 5-Hydroperoxide derivative of icosapentaenoic acid, which is produced concomitantly with action of 15-lipoxygenase against arachidonate, is further metabolized to hydroxylate and leukotrienes, but their physiological activities remain to be clarified Lee_TH et al.. | |
| Isomerization of hydroperoxides generated from icosapentaenoete by autooxidation: the proportion of 5-, 18-isomer (outer hydroperoxides) is higher than that of other isomer (8-,9-,11-,12-,14-,15-isomer[inner hydroperoxides]). | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(Me-ester; after reduction and TMS-derivatization) YamauchiRet al. OchiKet al. FurukawaMet al. HammarstromS: m/e=404[M]; 389[M-CH3]; 314[M-HOTMS]; 303[M-(CH2)3COOCH3]; 255[M-CH2CH=CH(CH2)3CH3]; 213[303-HOTMS]; 203[SMTO=CH(CH2)3COOCH3] |
| UV Spectra | UV(Me-ester) YamauchiRet al.: conjugated diene: λ max=235.5nm; UV(after reduction) HammarstromS: conjugated diene: λ =max=235nm |
| IR Spectra | IR(Me-ester) YamauchiRet al.: OOH group: 3400cm-1 |
| NMR Spectra | 1H-NMR YamauchiRet al.: OOH proton: 8.5ppm |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|