LBF20503SC01: Difference between revisions
No edit summary |
mNo edit summary |
||
| (7 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA0220 | |LipidBank=DFA0220 | ||
|LipidMaps=LMFA01030181 | |LipidMaps=LMFA01030181 | ||
|SysName=5, 8, 11, 14, 17-Eicosapentaenoic | |SysName=cis-5, cis-8, cis-11, cis-14, cis-17-Eicosapentaenoic acid | ||
|Common Name=&& | |Common Name=&&EPA&&5, 8, 11, 14, 17-Eicosapentaenoic acid&& | ||
|Melting Point=-54.4 to -53.8 °C | |Melting Point=-54.4 to -53.8 °C | ||
| | |Refractive=1.4977 at 23 °C | ||
|Solubility=soluble in heptane and methyl alcohol. | |Solubility=soluble in heptane and methyl alcohol.<!--0265--><!--0269--> | ||
|Mass Spectra=""METHYL ESTER:316,300,287,262,247,234,215,201,180,161,152 "" [[Reference:Karlsson_KA:Samuelsson_BE:Steen_GO:,Acta Chem. Scand.,1968,22,1361|{{RelationTable/GetFirstAuthor|Reference:Karlsson_KA:Samuelsson_BE:Steen_GO:,Acta Chem. Scand.,1968,22,1361}}]] | |Mass Spectra=""METHYL ESTER:316,300,287,262,247,234,215,201,180,161,152 "" [[Reference:Karlsson_KA:Samuelsson_BE:Steen_GO:,Acta Chem. Scand.,1968,22,1361|{{RelationTable/GetFirstAuthor|Reference:Karlsson_KA:Samuelsson_BE:Steen_GO:,Acta Chem. Scand.,1968,22,1361}}]] | ||
|NMR Spectra=""METHYL ESTER: | |NMR Spectra=""METHYL ESTER: | ||
| Line 16: | Line 16: | ||
|Source=Present in fish oils as an acylglycerol and animal phospholipids. Cattle-liver lipids; various fish and seal oils. | |Source=Present in fish oils as an acylglycerol and animal phospholipids. Cattle-liver lipids; various fish and seal oils. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism=Metabolic product of | |Metabolism=Metabolic product of alpha -linolenic acid (9,12,15-18:3). The synthesis of 5,8,11,14,17-20:5 (=EPA) occurs via the following reaction sequence in the endoplasmic reticulum [[Reference:Sprecher_H:Luthria_DL:Mohammed_BS:Baykousheva_SP:,J. Lipid. Res.,1995,36,2471|{{RelationTable/GetFirstAuthor|Reference:Sprecher_H:Luthria_DL:Mohammed_BS:Baykousheva_SP:,J. Lipid. Res.,1995,36,2471}}]]: 9,12,15-18:3 --> 6,9,12,15-18:4 --> 8,11,14,17-20:4 --> 5,8,11,14,17-20:5 --> 7,10,13,16,19-22:5. EPA is further metabolized to DHA. Precursor of PG3 series of prostaglandins. | ||
|Symbol=EPA / C20:5n-3 / C20:5 omega 3 | |||
|Biological Activity=Considered to be the major reason for the beneficial effects of fish oils on the cardiovascular system. EPA is a precursor to series 3 eicosanoids that promote dilation of blood vessels and a slower blood clotting reaction and, as such, has been found to be critical to the maintenance of normal cardiovascular health. [[Reference:Bang_HO:Dyerberg_J:,Acta Med. Scand.,1972,192,85|{{RelationTable/GetFirstAuthor|Reference:Bang_HO:Dyerberg_J:,Acta Med. Scand.,1972,192,85}}]][[Reference:Dyerberg_J:Bang_HO:Stoffersen_E:Moncada_S:Vane_JR:,Lancet,1978,2,117|{{RelationTable/GetFirstAuthor|Reference:Dyerberg_J:Bang_HO:Stoffersen_E:Moncada_S:Vane_JR:,Lancet,1978,2,117}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 00:13, 21 July 2016
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0220 |
| LipidMaps | LMFA01030181 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20503SC01 |
| EPA | |
|---|---|

| |
| Structural Information | |
| cis-5, cis-8, cis-11, cis-14, cis-17-Eicosapentaenoic acid | |
| |
| EPA / C20:5n-3 / C20:5 omega 3 | |
| Formula | C20H30O2 |
| Exact Mass | 302.224580204 |
| Average Mass | 302.451 |
| SMILES | C(CC=CCC=CCC=CCC=CCCCC(O)=O)=CCC |
| Physicochemical Information | |
| -54.4 to -53.8 °C | |
| 1.4977 at 23 °C | |
| soluble in heptane and methyl alcohol. | |
| Present in fish oils as an acylglycerol and animal phospholipids. Cattle-liver lipids; various fish and seal oils. | |
| Metabolic product of alpha -linolenic acid (9,12,15-18:3). The synthesis of 5,8,11,14,17-20:5 (=EPA) occurs via the following reaction sequence in the endoplasmic reticulum Sprecher_H et al.: 9,12,15-18:3 --> 6,9,12,15-18:4 --> 8,11,14,17-20:4 --> 5,8,11,14,17-20:5 --> 7,10,13,16,19-22:5. EPA is further metabolized to DHA. Precursor of PG3 series of prostaglandins. | |
| Considered to be the major reason for the beneficial effects of fish oils on the cardiovascular system. EPA is a precursor to series 3 eicosanoids that promote dilation of blood vessels and a slower blood clotting reaction and, as such, has been found to be critical to the maintenance of normal cardiovascular health. Bang_HO et al. Dyerberg_J et al. | |
| Spectral Information | |
| Mass Spectra | ""METHYL ESTER:316,300,287,262,247,234,215,201,180,161,152 "" Karlsson_KA et al. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | ""METHYL ESTER: |
| Other Spectra | |
| Chromatograms | Gas liquid chromatogram 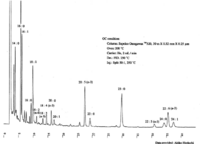 (provided by Dr. Akiko Horiuchi). |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|