LBF21503HO04: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA03070017 | |LipidMaps=LMFA03070017 | ||
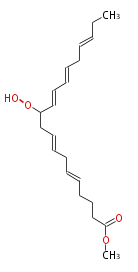
|SysName=Methyl 11-Hydroperoxy-5,8,12,14,17-Icosapentaenoate | |SysName=Methyl 11-Hydroperoxy-5,8,12,14,17-Icosapentaenoate | ||
|Mass Spectra=GC-EI-MS(after reduction and TMS-derivatization) | |Mass Spectra=GC-EI-MS(after reduction and TMS-derivatization)[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: m/e=404[M]; 389[M-CH3] 314[M-HOTMS], GC-EI-MS(Me-ester: after reduction, hydrogenation and TMS-derivatization) [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: m/e=399[M-CH3]; 383[M-OCH3]; 367[399-CH3OH] | ||
|UV Spectra=conjugated diene: <FONT FACE="Symbol">l</FONT>max=235.5nm | |UV Spectra=conjugated diene: <FONT FACE="Symbol">l</FONT>max=235.5nm [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]] | ||
|IR Spectra=OOH group: 3400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=OOH group: 3400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: OOH proton: 8.5ppm | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8100 |
| LipidMaps | LMFA03070017 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF21503HO04 |
| GlcNAca/b1-3Xyla-4Galb1-3GalNAca1-4(NeuAc?1-2NeuGc4Mea1-3)GalNAcb1-4(EtnP-6)GlcNAcb1-3Manb1-4Glcb1-1Cer | |
|---|---|
| |
| Structural Information | |
| Methyl 11-Hydroperoxy-5,8,12,14,17-Icosapentaenoate | |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after reduction and TMS-derivatization) YamauchiRet al.: m/e=404[M]; 389[M-CH3] 314[M-HOTMS], GC-EI-MS(Me-ester: after reduction, hydrogenation and TMS-derivatization) YamauchiRet al.: m/e=399[M-CH3]; 383[M-OCH3]; 367[399-CH3OH] |
| UV Spectra | conjugated diene: lmax=235.5nm YamauchiRet al. |
| IR Spectra | OOH group: 3400cm-1 YamauchiRet al. |
| NMR Spectra | 1H-NMR YamauchiRet al.: OOH proton: 8.5ppm |
| Other Spectra | |
| Chromatograms | |