LBF21503HO04: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
|IR Spectra=OOH group: 3400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]] | |IR Spectra=OOH group: 3400cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: OOH proton: 8.5ppm | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: OOH proton: 8.5ppm | ||
|Source=Autooxidation of icosapentaenoate[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]];>. Oxidation of icosapentaenoate by singlet-oxygen[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]];>. Oxidation of icosapentaenoate in the presence of myoglobin. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 22:00, 24 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8100 |
| LipidMaps | LMFA03070017 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF21503HO04 |
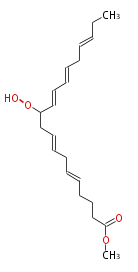
| Methyl 11-Hydroperoxy-5,8,12,14,17-Icosapentaenoate | |
|---|---|
| |
| Structural Information | |
| Methyl 11-Hydroperoxy-5,8,12,14,17-Icosapentaenoate | |
| |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| Autooxidation of icosapentaenoate Yamauchi_R et al.;>. Oxidation of icosapentaenoate by singlet-oxygen Yamauchi_R et al.;>. Oxidation of icosapentaenoate in the presence of myoglobin. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after reduction and TMS-derivatization) YamauchiRet al.: m/e=404[M]; 389[M-CH3] 314[M-HOTMS], GC-EI-MS(Me-ester: after reduction, hydrogenation and TMS-derivatization) YamauchiRet al.: m/e=399[M-CH3]; 383[M-OCH3]; 367[399-CH3OH] |
| UV Spectra | conjugated diene: lmax=235.5nm YamauchiRet al. |
| IR Spectra | OOH group: 3400cm-1 YamauchiRet al. |
| NMR Spectra | 1H-NMR YamauchiRet al.: OOH proton: 8.5ppm |
| Other Spectra | |
| Chromatograms | |