LBF21503HO04: Difference between revisions
No edit summary |
No edit summary |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 6: | Line 6: | ||
|LipidBank=DFA8100 | |LipidBank=DFA8100 | ||
|LipidMaps=LMFA03070017 | |LipidMaps=LMFA03070017 | ||
|SysName=Methyl 11- | |SysName=Methyl-11-hydroperoxy-5,8,12,14,17-eicosapentaenoic acid | ||
|Common Name=&&Methyl 11- | |Common Name=&&Methyl-11-hydroperoxy-5,8,12,14,17-eicosapentaenoic acid&& | ||
|Mass Spectra=GC-EI-MS(after reduction and TMS-derivatization)[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: m/e=404[M]; 389[M-CH3] 314[M-HOTMS], GC-EI-MS(Me-ester: after reduction, hydrogenation and TMS-derivatization) [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: m/e=399[M-CH3]; 383[M-OCH3]; 367[399-CH3OH] | |Mass Spectra=GC-EI-MS(after reduction and TMS-derivatization)[[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: m/e=404[M]; 389[M-CH3] 314[M-HOTMS], GC-EI-MS(Me-ester: after reduction, hydrogenation and TMS-derivatization) [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]]: m/e=399[M-CH3]; 383[M-OCH3]; 367[399-CH3OH] | ||
|UV Spectra=conjugated diene: lambda max=235.5nm [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]] | |UV Spectra=conjugated diene: lambda max=235.5nm [[Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897|{{RelationTable/GetFirstAuthor|Reference:Yamauchi_R:Yamada_T:Kato_K:Ueno_Y:,Agric. Biol. Chem.,1983,47,2897}}]] | ||
Latest revision as of 08:59, 2 June 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8100 |
| LipidMaps | LMFA03070017 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF21503HO04 |
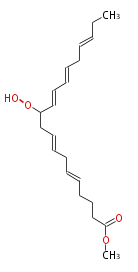
| Methyl-11-hydroperoxy-5,8,12,14,17-eicosapentaenoic acid | |
|---|---|
| |
| Structural Information | |
| Methyl-11-hydroperoxy-5,8,12,14,17-eicosapentaenoic acid | |
| |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| Autooxidation of icosapentaenoate Yamauchi_R et al.. Oxidation of icosapentaenoate by singlet-oxygen Yamauchi_R et al.. Oxidation of icosapentaenoate in the presence of myoglobin. | |
| Isomerization of hydroperoxides generated from icosapentaenoete by autooxidation: the proportion of 5-, 18-isomer (outer hydroperoxides) is higher than that of other isomer (8-,9-,11-,12-,14-,15-isomer[inner hydroperoxides]). | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after reduction and TMS-derivatization) YamauchiRet al.: m/e=404[M]; 389[M-CH3] 314[M-HOTMS], GC-EI-MS(Me-ester: after reduction, hydrogenation and TMS-derivatization) YamauchiRet al.: m/e=399[M-CH3]; 383[M-OCH3]; 367[399-CH3OH] |
| UV Spectra | conjugated diene: λ max=235.5nm YamauchiRet al. |
| IR Spectra | OOH group: 3400cm-1 YamauchiRet al. |
| NMR Spectra | 1H-NMR YamauchiRet al.: OOH proton: 8.5ppm |
| Other Spectra | |
| Chromatograms | |