LBF20406LT03: Difference between revisions
No edit summary |
No edit summary |
||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 4: | Line 6: | ||
|LipidBank=XPR3111 | |LipidBank=XPR3111 | ||
|LipidMaps=LMFA03020024 | |LipidMaps=LMFA03020024 | ||
|SysName=5S- | |SysName=5S-12-Keto- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenoic acid | ||
|Common Name=&&12-oxo-Leukotriene | |Common Name=&&12-oxo-Leukotriene B_4&&5S-12-Keto- (6Z,8E,10E,14Z) -eicosatraenoic acid&& | ||
|Solubility=Soluble in ethanol, methanol, ethyl acetate, acetonitril | |Solubility=Soluble in ethanol, methanol, ethyl acetate, acetonitril | ||
|UV Spectra=UV maxima 316 nm, Absorbance at 320 nm is 41000/M | |UV Spectra=UV maxima 316 nm, Absorbance at 320 nm is 41000/M | ||
|Chromatograms=12-oxo-LTB4 is eluted at 7.8 min on RP-HPLC system as follows: Solvent:acetonitril/water/acetic acid, 50:50:0.01 (v/v/v), 0.01 % (w/v) Na2EDTA, pH 5.6 with ammonia Flow: 1 ml/min, isocratic Column: Cosmosil 5C18-AR (4.6 x 150 mm, Nacalai tesque, Tokyo) | |Chromatograms=12-oxo-LTB4 is eluted at 7.8 min on RP-HPLC system as follows: Solvent:acetonitril/water/acetic acid, 50:50:0.01 (v/v/v), 0.01 % (w/v) Na2EDTA, pH 5.6 with ammonia Flow: 1 ml/min, isocratic Column: Cosmosil 5C18-AR (4.6 x 150 mm, Nacalai tesque, Tokyo) [[Reference:Yokomizo_T:Izumi_T:Takahashi_T:Kasama_T:Kobayashi_Y:Sato_F:Taketani_Y:Shimizu_T:,J. Biol. Chem.,1993,268,18128|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Izumi_T:Takahashi_T:Kasama_T:Kobayashi_Y:Sato_F:Taketani_Y:Shimizu_T:,J. Biol. Chem.,1993,268,18128}}]] | ||
|Source=This metabolite is derived from LTB4 by LTB4 12-hydroxydehydrogenase. LTB4 12-hydroxydehydrogenase is expressed most abunduntly in liver and kidney. | |||
|Chemical Synthesis= | |||
|Metabolism= | |||
|Symbol=12-oxo-LTB4 | |||
|Biological Activity=Increase in intracellular calcium in human leukocytes probably through BLT (LTB4 receptor). The IC 50 value is 100 times higher than that of LTB4. | |||
|Genetic Information=LTB4 12-hydroxydehydrogenase cDNA is cloned from various animals. The accession numbers in DDBJ/EMBL/Genbank are shown in ( ). Pig (D49386) [[Reference:Yokomizo_T:Ogawa_Y:Uozumi_N:Kume_K:Izumi_T:Shimizu_T:,J. Biol. Chem.,1996,271,2844|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Ogawa_Y:Uozumi_N:Kume_K:Izumi_T:Shimizu_T:,J. Biol. Chem.,1996,271,2844}}]] [[Reference:Ensor_CM:Zhang_H:Tai_HH:,Biochem. J.,1998,330 (Pt 1),103|{{RelationTable/GetFirstAuthor|Reference:Ensor_CM:Zhang_H:Tai_HH:,Biochem. J.,1998,330 (Pt 1),103}}]] Human (D49387) [[Reference:Yokomizo_T:Ogawa_Y:Uozumi_N:Kume_K:Izumi_T:Shimizu_T:,J. Biol. Chem.,1996,271,2844|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Ogawa_Y:Uozumi_N:Kume_K:Izumi_T:Shimizu_T:,J. Biol. Chem.,1996,271,2844}}]] Rat (U66322) [[Reference:Primiano_T:Gastel_JA:Kensler_TW:Sutter_TR:,Carcinogenesis,1996,17,2297|{{RelationTable/GetFirstAuthor|Reference:Primiano_T:Gastel_JA:Kensler_TW:Sutter_TR:,Carcinogenesis,1996,17,2297}}]] | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 07:50, 21 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3111 |
| LipidMaps | LMFA03020024 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT03 |
| 12-oxo-Leukotriene B4 | |
|---|---|
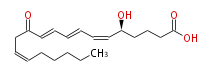
| |
| Structural Information | |
| 5S-12-Keto- (cis-6,trans-8,trans-10,cis-14) -eicosatetraenoic acid | |
| |
| 12-oxo-LTB4 | |
| Formula | C20H30O4 |
| Exact Mass | 334.21440944799997 |
| Average Mass | 334.4498 |
| SMILES | C(CC=CCC(C=CC=CC=C[C@H](CCCC(O)=O)O)=O)CCC |
| Physicochemical Information | |
| Soluble in ethanol, methanol, ethyl acetate, acetonitril | |
| This metabolite is derived from LTB4 by LTB4 12-hydroxydehydrogenase. LTB4 12-hydroxydehydrogenase is expressed most abunduntly in liver and kidney. | |
| Increase in intracellular calcium in human leukocytes probably through BLT (LTB4 receptor). The IC 50 value is 100 times higher than that of LTB4. | |
| LTB4 12-hydroxydehydrogenase cDNA is cloned from various animals. The accession numbers in DDBJ/EMBL/Genbank are shown in ( ). Pig (D49386) Yokomizo_T et al. Ensor_CM et al. Human (D49387) Yokomizo_T et al. Rat (U66322) Primiano_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | UV maxima 316 nm, Absorbance at 320 nm is 41000/M |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | 12-oxo-LTB4 is eluted at 7.8 min on RP-HPLC system as follows: Solvent:acetonitril/water/acetic acid, 50:50:0.01 (v/v/v), 0.01 % (w/v) Na2EDTA, pH 5.6 with ammonia Flow: 1 ml/min, isocratic Column: Cosmosil 5C18-AR (4.6 x 150 mm, Nacalai tesque, Tokyo) YokomizoTet al. |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|