LBF20407HO04: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
{{Metabolite | {{Metabolite | ||
|LipidBank=DFA8105 | |LipidBank=DFA8105 | ||
|LipidMaps= | |LipidMaps=LMFA03060050 | ||
|SysName=8S,15S-dihydroxy-5Z,9E,11Z,13E-eicosatetraenoic acid | |SysName=8S,15S-dihydroxy-5Z,9E,11Z,13E-eicosatetraenoic acid | ||
|Common Name=&&8S,15S-dihydroxy-5Z,9E,11Z,13E-eicosatetraenoic acid&& | |Common Name=&&8S,15S-dihydroxy-5Z,9E,11Z,13E-eicosatetraenoic acid&& | ||
Revision as of 02:59, 12 March 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8105 |
| LipidMaps | LMFA03060050 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20407HO04 |
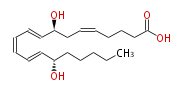
| 8S,15S-dihydroxy-5Z,9E,11Z,13E-eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 8S,15S-dihydroxy-5Z,9E,11Z,13E-eicosatetraenoic acid | |
| |
| 8(S),15(S)-DiHETE | |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CCC(C=CC=CC=CC(CC=CCCCC(O)=O)O)O)CC |
| Physicochemical Information | |
| 8(S),15(S)-DiHETE is formed when15(S)-HETE is subjected to further oxidation by 15-lipoxygenase Morita_E et al.. | |
| 8(S),15(S)-diHETE causes chemotaxis of human eosinophils with an ED _{50} of 1.5 mu M but is not chemotactic for neutrophils Morita_E et al.. 8(S),15(S)-diHETE exhibits hypoalgesic activity by antagonizing the hyperalgesic activity of 8(R),15(S)-diHETE and LTB 4 when measuring nociceptive activity in the rat hindpaw model of pain Oliw_EH . | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 269 nm ε : 40,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|