LBGPEc-p:R::YS2ANe005: Difference between revisions
m (LBGPEc-p:R::YS2ANe005:01 moved to LBGPEc-p:R::YS2ANe005) |
m (LBGPEc-p:R::YS2ANe005:01 moved to LBGPEc-p:R::YS2ANe005) |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
|LipidBank=PGP2416 | |LipidBank=PGP2416 | ||
|SysName=1-acyl-sn-glycero- (3) -phosphoethanolamine | |SysName=1-acyl-sn-glycero- (3) -phosphoethanolamine | ||
|Common Name=&&lysophosphatidylethanolamine&&1-acyl | |Common Name=&&lysophosphatidylethanolamine&&1-acyl&& | ||
|Melting Point=205° <<2520>>; 1-palmitoyl-DL-lysoPE, 212°C <<2517>> | |Melting Point=205° <<2520>>; 1-palmitoyl-DL-lysoPE, 212°C <<2517>> | ||
|Chromatograms=Two-dimensional thin-layer chromatography. Silica gel 60 plate (Merch, Germany) was done in the first developement solution, composed of tetrahydrofuran-acetone-methanol-water (50: 20: 40: 8, by volume), and in the second developement solution, composed of chloroform-acetone-methanol-acetic acid-water (50: 20: 10: 15: 5, by volume) {{Image200|LBGPEc-p:R:: | |Chromatograms=Two-dimensional thin-layer chromatography. Silica gel 60 plate (Merch, Germany) was done in the first developement solution, composed of tetrahydrofuran-acetone-methanol-water (50: 20: 40: 8, by volume), and in the second developement solution, composed of chloroform-acetone-methanol-acetic acid-water (50: 20: 10: 15: 5, by volume) {{Image200|LBGPEc-p:R::YS2ANe005CH0001.gif}} <<2521>>./<BR>HPLC: PE,dipalmitoyl-phosphatidylethanolamine; LPE,1-palmitoyl-glycerophosphorylethanolamine; PC,dipalmitoyl-phosphatidylcholine; LPC,1-palmitoyl-glycerophosphorylcholine {{Image200|LBGPEc-p:R::YS2ANe005CH0002.gif}} <<2522>>., | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 21:00, 14 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | PGP2416 |
| LipidMaps | [1] |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBGPEc-p:R::YS2ANe005 |
| lysophosphatidylethanolamine | |
|---|---|
| |
| Structural Information | |
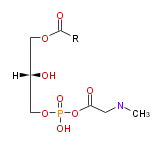
| 1-acyl-sn-glycero- (3) -phosphoethanolamine | |
| |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| 205° <<2520>>; 1-palmitoyl-DL-lysoPE, 212°C <<2517>> | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | Two-dimensional thin-layer chromatography. Silica gel 60 plate (Merch, Germany) was done in the first developement solution, composed of tetrahydrofuran-acetone-methanol-water (50: 20: 40: 8, by volume), and in the second developement solution, composed of chloroform-acetone-methanol-acetic acid-water (50: 20: 10: 15: 5, by volume) 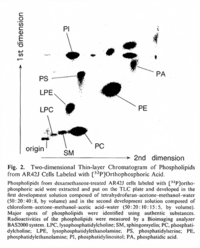 <<2521>>./ HPLC: PE,dipalmitoyl-phosphatidylethanolamine; LPE,1-palmitoyl-glycerophosphorylethanolamine; PC,dipalmitoyl-phosphatidylcholine; LPC,1-palmitoyl-glycerophosphorylcholine 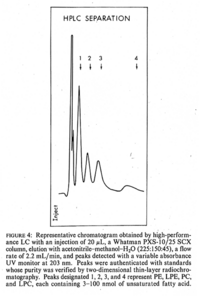 <<2522>>., |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|