LBGPEccp:R:R:YB2ANe001: Difference between revisions
m (LBGPEccp:R:R:YB2ANe001:01 moved to LBGPEccp:R:R:YB2ANe001) |
m (LBGPEccp:R:R:YB2ANe001:01 moved to LBGPEccp:R:R:YB2ANe001) |
||
| Line 4: | Line 4: | ||
|LipidBank=PGP2401 | |LipidBank=PGP2401 | ||
|SysName=1,2-di-acyl-sn-glycero- (3) -phospho-N-methylethanolamine | |SysName=1,2-di-acyl-sn-glycero- (3) -phospho-N-methylethanolamine | ||
|Common Name=phosphatidyl-N-methylethanolamine N- | |Common Name=&&phosphatidyl-N-methylethanolamine&& N-methylphosphatidylethanolamine&&phosphatidyl-N-monomethylethanolamine&&1,2-di-acyl-sn-glycero- (3) -phospho-N-methylethanolamine&& | ||
|Melting Point=1,2-distearoyl-L-N-methylphosphatidylethanolamine, 178-180°C 2412; 1,2-distearoyl-DL-N-methylphosphatidylethanolamine, 171-172°C 2412; 1,2-dipalmitoyl-L-N-methylphosphatidylethanolamine, 177-178°C 2412, 184-186°C (from | |Melting Point=1,2-distearoyl-L-N-methylphosphatidylethanolamine, 178-180°C <<2412>>; 1,2-distearoyl-DL-N-methylphosphatidylethanolamine, 171-172°C <<2412>>; 1,2-dipalmitoyl-L-N-methylphosphatidylethanolamine, 177-178°C <<2412>>, 184-186°C (from CHCl<SUB><FONT SIZE=-1>3</FONT></SUB>-MeOH at 4°C <<2413>>; 1,2-dipalmitoyl-DL-N-methylphosphatidylethanolamine, 170-171°C <<2412>>, 182°C <<2413>> | ||
|Reflactive=1,2-distearoyl-L-N-methylphosphatidylethanolamine, [FONT FACE= | |Reflactive=1,2-distearoyl-L-N-methylphosphatidylethanolamine, [<FONT FACE="Symbol">a</FONT>]<sub>D</sub><sup>25</sup> +7.1° <<2412>>; 1,2-dipalmitoyl-L-N-methylphosphatidylethanolamine, [<FONT FACE="Symbol">a</FONT>]<sub>D</sub><sup>25</sup> +8.0° <<2412>>, [<FONT FACE="Symbol">a</FONT>]<sub>D</sub><sup>23</sup> +8.0° (in CHCl<SUB><FONT SIZE=-1>3</FONT></SUB>) <<2413>> | ||
|NMR Spectra= | |NMR Spectra=<SUP><FONT SIZE=-1>2</FONT></SUP>H-NMR spectra of PC, PDME, PMME, and PE fully hydrated with D<SUB><FONT SIZE=-1>2</FONT></SUB>O at the indicated temperatures {{Image200|LBGPEccp:R:R:YB2ANe001SP0001.gif}} <<2410>>., | ||
|Chromatograms=HPLC, FONT FACE= | |Chromatograms=HPLC, <FONT FACE="Symbol">m</FONT>Bondapak NH<SUB><FONT SIZE=-1>2</FONT></SUB>, acetonitril-methanol-water (13:7:1, v/v), elution rate 1.6 ml/min, detection OD<SUB><FONT SIZE=-1>2</FONT></SUB><SUB><FONT SIZE=-1>0</FONT></SUB><SUB><FONT SIZE=-1>3</FONT></SUB> nm, Peaks: SF = solvent front, PC = phosphatidylcholine, SPH = sphingomyelin, LPC = lysophosphatidylcholine, PMME = phosphatidylmonomethylethanolamine, PE = phosphatidylethanolamine, PDME = phosphatidyldimethylethanolamine, LPE = lysophosphatidylethanolamine, {{Image200|LBGPEccp:R:R:YB2ANe001CH0001.gif}} <<2401>>/<BR>TLC, plate: Silica gel 60 (Merck, 20 X 20 cm, 0.25 mm thick), solvent: 1st dim. chloroform - methanol - water (65:25:4, v/v), 2nd dim. n-butanol - acetic acid - water (6:2:2, v/v) <<2402>>, | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 15:00, 18 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | PGP2401 |
| LipidMaps | [1] |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBGPEccp:R:R:YB2ANe001 |
| phosphatidyl-N-methylethanolamine | |
|---|---|
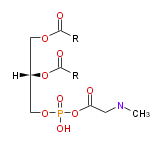
| |
| Structural Information | |
| 1,2-di-acyl-sn-glycero- (3) -phospho-N-methylethanolamine | |
| |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| 1,2-distearoyl-L-N-methylphosphatidylethanolamine, 178-180°C <<2412>>; 1,2-distearoyl-DL-N-methylphosphatidylethanolamine, 171-172°C <<2412>>; 1,2-dipalmitoyl-L-N-methylphosphatidylethanolamine, 177-178°C <<2412>>, 184-186°C (from CHCl3-MeOH at 4°C <<2413>>; 1,2-dipalmitoyl-DL-N-methylphosphatidylethanolamine, 170-171°C <<2412>>, 182°C <<2413>> | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 2H-NMR spectra of PC, PDME, PMME, and PE fully hydrated with D2O at the indicated temperatures 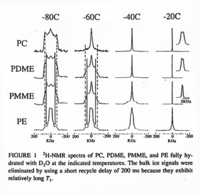 <<2410>>., |
| Other Spectra | |
| Chromatograms | HPLC, mBondapak NH2, acetonitril-methanol-water (13:7:1, v/v), elution rate 1.6 ml/min, detection OD203 nm, Peaks: SF = solvent front, PC = phosphatidylcholine, SPH = sphingomyelin, LPC = lysophosphatidylcholine, PMME = phosphatidylmonomethylethanolamine, PE = phosphatidylethanolamine, PDME = phosphatidyldimethylethanolamine, LPE = lysophosphatidylethanolamine, 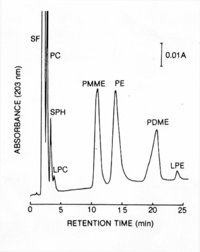 <<2401>>/ TLC, plate: Silica gel 60 (Merck, 20 X 20 cm, 0.25 mm thick), solvent: 1st dim. chloroform - methanol - water (65:25:4, v/v), 2nd dim. n-butanol - acetic acid - water (6:2:2, v/v) <<2402>>, |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|