LBGPEccp:R:R:YS2ANe005: Difference between revisions
m (LBGPEccp:R:R:YS2ANe005:01 moved to LBGPEccp:R:R:YS2ANe005) |
m (LBGPEccp:R:R:YS2ANe005:01 moved to LBGPEccp:R:R:YS2ANe005) |
||
| Line 6: | Line 6: | ||
|Common Name=&&phosphatidylethanolamine&& cephalin&& ethanolaminephosphoglyceride&& (3-sn-phosphatidyl) ethanolamine &&L-a-phosphatidylethanolamine&& | |Common Name=&&phosphatidylethanolamine&& cephalin&& ethanolaminephosphoglyceride&& (3-sn-phosphatidyl) ethanolamine &&L-a-phosphatidylethanolamine&& | ||
|Melting Point=196°C <<2455>>; dimyristoyl-PE, 175-177°C <<2456/2457>>; dipalmitoyl-PE, 172-175°C <<2456/2457>> 210-211°C <<2469>> dipalmitoyl-DL-, 211°C <<2469>>; distearoyl-PE, 172-173.5°C <<2456/2457>>; dioleoyl-PE, 195-200°C <<2469>>; dielaidoyl-PE, 193°C <<2469>>; 1-palmitoyl-2-oleoyl-PE, 194-196°C <<2471>> | |Melting Point=196°C <<2455>>; dimyristoyl-PE, 175-177°C <<2456/2457>>; dipalmitoyl-PE, 172-175°C <<2456/2457>> 210-211°C <<2469>> dipalmitoyl-DL-, 211°C <<2469>>; distearoyl-PE, 172-173.5°C <<2456/2457>>; dioleoyl-PE, 195-200°C <<2469>>; dielaidoyl-PE, 193°C <<2469>>; 1-palmitoyl-2-oleoyl-PE, 194-196°C <<2471>> | ||
|Reflactive=dimyristoyl-PE , [ | |Reflactive=dimyristoyl-PE , [alpha]^26_D +6.7° in CHCl_3 <<2456/2457>>; dipalmitoyl-PE, [alpha]^26_D +6.4° in CHCl_3 <<2456/2457>>, [alpha]^25_D +6.2° in CHCl_3 -MeOH (2:1,v/v) <<2469>>; distearoyl-PE, [alpha]^24_D +6.0° in CHCl_3 -glacial acetic acid (9:1,v/v) <<2456/2457>>; dioleoyl-PE, [alpha]^22_D +6.0° in CHCl_3 <<2469>>; dielaidoyl-PE, [alpha]^22_D +6.1° in CHCl_3 <<2469>>; 1-palmitoyl-2-oleoyl-PE, [alpha]^22_D +6.35° in CHCl_3 <<2469>> | ||
|Solubility=Insoluble in water. Natural PE is soluble in MeOH, EtOH, | |Solubility=Insoluble in water. Natural PE is soluble in MeOH, EtOH, CHCl_3 , benzene, ether, and petroleum ether, and insoluble in acetone. At 20°C, synthetic PEs are insoluble (<=1mg/100 ml of dry solvent) in acetone, ether, petroleum ether, and ethyl acetate; moderately soluble (20-100 mg/100 ml of dry solvent) in EtOH, pyridine, benzene, and CCl_4 ; readily soluble (> 1g/100 ml of solvent) in CHCl_3 <<2455/2456/2457>>. | ||
|Mass Spectra=Negative ion mass spectrum during the elution of 1-stearol-2-arachidonyl-PE in a sample of rat liver PE. A Fisons VG Platform II single quadrupole mass spectrometer, fitted with an electrospray ion source operated at atmospheric pressure, was used for the identification of components eluting from the column. Nitrogen was used as nebulizer gas and as curtain gas. The capillary voltage was set to 3.0 kV and the cone voltage to 100 V {{Image200|LBGPEccp:R:R:YS2ANe005SP0004.gif}} <<2462>>., | |Mass Spectra=Negative ion mass spectrum during the elution of 1-stearol-2-arachidonyl-PE in a sample of rat liver PE. A Fisons VG Platform II single quadrupole mass spectrometer, fitted with an electrospray ion source operated at atmospheric pressure, was used for the identification of components eluting from the column. Nitrogen was used as nebulizer gas and as curtain gas. The capillary voltage was set to 3.0 kV and the cone voltage to 100 V {{Image200|LBGPEccp:R:R:YS2ANe005SP0004.gif}} <<2462>>., | ||
|UV Spectra=Maximum absorbance at 205 nm <<2458>>. | |UV Spectra=Maximum absorbance at 205 nm <<2458>>. | ||
|IR Spectra=Fourier transform IR spectra for dimyristoyl-PE in the dry state (anhydrous), either in the pure state (A, C and E) or in the presence of 20 mol% of | |IR Spectra=Fourier transform IR spectra for dimyristoyl-PE in the dry state (anhydrous), either in the pure state (A, C and E) or in the presence of 20 mol% of alpha-tocopherol (B, D and F). Ester C=O stretching vibration mode region,1800-1700 cm^- ^1 ; CH_2^- ^1 ; headgroup PO_2^- phosphate band region, 1350-1150 cm^- ^1 {{Image200|LBGPEccp:R:R:YS2ANe005SP0001.gif}} <<2459>>. | ||
|NMR Spectra= | |NMR Spectra=^1 H-NMR spectrum of PE. Varian VXR500 spectrometer operating at 500 MHz for protons, using CHCl_3 as the lock signal {{Image200|LBGPEccp:R:R:YS2ANe005SP0002.gif}} <<2460>>./<BR>^3 ^1 P NMR spectrum of a PE (left) and a PC (right) fraction from bovine brain. Varian Unity 300 spectrometer operating at121.42 MHz using 1% H_3 PO_4 as external standard {{Image200|LBGPEccp:R:R:YS2ANe005SP0003.gif}} <<2461>>., | ||
|Chromatograms=2 dimensional TLC {{Image200|LBGPEccp:R:R:YS2ANe005CH0001.gif}} <<2463>>/<BR>HPLC, Hewlett-Packard Model 1050 HPLC system, UV absorbance 205nm, Hewlett-Packard Model 3396 series II integrator, HP Hypersil C18 (200 x 2.1x 5 | |Chromatograms=2 dimensional TLC {{Image200|LBGPEccp:R:R:YS2ANe005CH0001.gif}} <<2463>>/<BR>HPLC, Hewlett-Packard Model 1050 HPLC system, UV absorbance 205nm, Hewlett-Packard Model 3396 series II integrator, HP Hypersil C18 (200 x 2.1x 5 mum) column {{Image200|LBGPEccp:R:R:YS2ANe005CH0002.gif}} <<2464>>/<BR> HPLC for intact PE molecular species. Molecular species of approxymately 50 nmol of PE were separated on two 250 x 4 mm Lichrospher RP-18 endcapped columns in series (Merck, Darmstadt, Germany). Isocratic elution was performed with a solvent consisting of acetonitrile-methanol 3:7 (v/v) containing 5 muM ethanolamine, or a solvent consisiting of acetonitrile-methanol 2:3 (v/v) containing 2 muM ethanolamine at a flow rate of 1.25 ml/min. The column effuluent was monitored at 206 nm using LKB 2251 Uvicord (Pharmacia, Upsala, Sweden) and subsequent ELSD was performed using a Varex MKIII obtained from Alltech, (Deerfield, IL) operating at a gas flow rate of 1.9 l/ml and a drift tube temperature of 100°C {{Image200|LBGPEccp:R:R:YS2ANe005CH0003.gif}} {{Image200|LBGPEccp:R:R:YS2ANe005FT0001.gif}} <<2462>>., | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 14:00, 19 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | PGP2410 |
| LipidMaps | [1] |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBGPEccp:R:R:YS2ANe005 |
| phosphatidylethanolamine | |
|---|---|
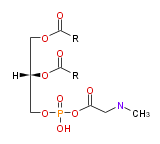
| |
| Structural Information | |
| L-a-phosphatidylethanolamine | |
| |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| 196°C <<2455>>; dimyristoyl-PE, 175-177°C <<2456/2457>>; dipalmitoyl-PE, 172-175°C <<2456/2457>> 210-211°C <<2469>> dipalmitoyl-DL-, 211°C <<2469>>; distearoyl-PE, 172-173.5°C <<2456/2457>>; dioleoyl-PE, 195-200°C <<2469>>; dielaidoyl-PE, 193°C <<2469>>; 1-palmitoyl-2-oleoyl-PE, 194-196°C <<2471>> | |
| Insoluble in water. Natural PE is soluble in MeOH, EtOH, CHCl3, benzene, ether, and petroleum ether, and insoluble in acetone. At 20°C, synthetic PEs are insoluble (<=1mg/100 ml of dry solvent) in acetone, ether, petroleum ether, and ethyl acetate; moderately soluble (20-100 mg/100 ml of dry solvent) in EtOH, pyridine, benzene, and CCl4; readily soluble (> 1g/100 ml of solvent) in CHCl3 <<2455/2456/2457>>. | |
| Spectral Information | |
| Mass Spectra | Negative ion mass spectrum during the elution of 1-stearol-2-arachidonyl-PE in a sample of rat liver PE. A Fisons VG Platform II single quadrupole mass spectrometer, fitted with an electrospray ion source operated at atmospheric pressure, was used for the identification of components eluting from the column. Nitrogen was used as nebulizer gas and as curtain gas. The capillary voltage was set to 3.0 kV and the cone voltage to 100 V 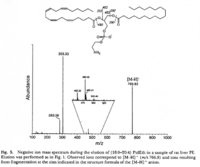 <<2462>>., |
| UV Spectra | Maximum absorbance at 205 nm <<2458>>. |
| IR Spectra | Fourier transform IR spectra for dimyristoyl-PE in the dry state (anhydrous), either in the pure state (A, C and E) or in the presence of 20 mol% of α-tocopherol (B, D and F). Ester C=O stretching vibration mode region,1800-1700 cm-1; CH_2-1; headgroup PO_2- phosphate band region, 1350-1150 cm-1 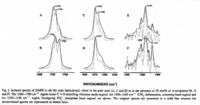 <<2459>>. |
| NMR Spectra | 1H-NMR spectrum of PE. Varian VXR500 spectrometer operating at 500 MHz for protons, using CHCl3 as the lock signal 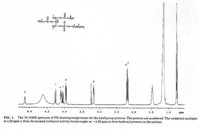 <<2460>>./ 31P NMR spectrum of a PE (left) and a PC (right) fraction from bovine brain. Varian Unity 300 spectrometer operating at121.42 MHz using 1% H3PO4 as external standard 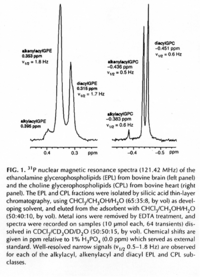 <<2461>>., |
| Other Spectra | |
| Chromatograms | 2 dimensional TLC 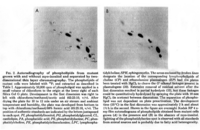 <<2463>>/ HPLC, Hewlett-Packard Model 1050 HPLC system, UV absorbance 205nm, Hewlett-Packard Model 3396 series II integrator, HP Hypersil C18 (200 x 2.1x 5 mum) column 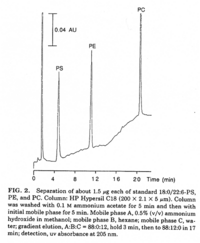 <<2464>>/ HPLC for intact PE molecular species. Molecular species of approxymately 50 nmol of PE were separated on two 250 x 4 mm Lichrospher RP-18 endcapped columns in series (Merck, Darmstadt, Germany). Isocratic elution was performed with a solvent consisting of acetonitrile-methanol 3:7 (v/v) containing 5 muM ethanolamine, or a solvent consisiting of acetonitrile-methanol 2:3 (v/v) containing 2 muM ethanolamine at a flow rate of 1.25 ml/min. The column effuluent was monitored at 206 nm using LKB 2251 Uvicord (Pharmacia, Upsala, Sweden) and subsequent ELSD was performed using a Varex MKIII obtained from Alltech, (Deerfield, IL) operating at a gas flow rate of 1.9 l/ml and a drift tube temperature of 100°C 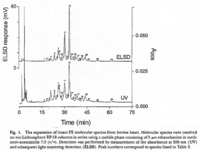 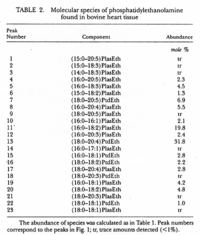 <<2462>>., |