LBGPEccp:R:R:p: Difference between revisions
m (LBGPEccp:R:R:ppppppppp:01 moved to LBGPEccp:R:R:p) |
m (LBGPEccp:R:R:ppppppppp:01 moved to LBGPEccp:R:R:p) |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
|LipidBank=PGP2404 | |LipidBank=PGP2404 | ||
|SysName=1,2-di-acyl-sn-glycero- (3) -phospho-N,N-dimethylethanolamine | |SysName=1,2-di-acyl-sn-glycero- (3) -phospho-N,N-dimethylethanolamine | ||
|Common Name=phosphatidyl-N,N- | |Common Name=&&phosphatidyl-N,N-dimethylethanolamine&&N,N-dimethylphosphatidylethanolamine&& | ||
|Melting Point=1,2-distearoyl-L-N,N-dimethylphosphatidylethanolamine, 168-169°C; 1,2-distearoyl-DL-N,N-dimethylphosphatidylethanolamine, 160-162°C; 1,2-dipalmitoyl-L-N,N-dimethylphosphatidylethanolamine, 164-165°C; 1,2-dipalmitoyl-DL-N,N-dimethylphosphatidylethanolamine, 160-161°C 2437 | |Melting Point=1,2-distearoyl-L-N,N-dimethylphosphatidylethanolamine, 168-169°C; 1,2-distearoyl-DL-N,N-dimethylphosphatidylethanolamine, 160-162°C; 1,2-dipalmitoyl-L-N,N-dimethylphosphatidylethanolamine, 164-165°C; 1,2-dipalmitoyl-DL-N,N-dimethylphosphatidylethanolamine, 160-161°C <<2437>> | ||
|Reflactive=1,2-distearoyl-L-N-methylphosphatidylethanolamine, [ | |Reflactive=1,2-distearoyl-L-N-methylphosphatidylethanolamine, [ alpha ]^{25}_D +5.8°; 1,2-dipalmitoyl-L-N-methylphosphatidylethanolamine, [ alpha ]^{25}_D +5.8° <<2437>> | ||
|NMR Spectra= | |NMR Spectra=^2 H-NMR spectra of PC, PDME, PMME, and PE fully hydrated with D_2 O at the indicated temperatures {{Image200|LBGPEccp:R:R:pSP0001.gif}} <<2435>>., | ||
|Chromatograms=HPLC, | |Chromatograms=HPLC, mu Bondapak NH_2 , acetonitril-methanol-water (13:7:1, v/v), elution rate 1.6 ml/min, detection OD_{203} nm, Peaks: SF = solvent front, PC = phosphatidylcholine, SPH = sphingomyelin, LPC = lysophosphatidylcholine, PMME = phosphatidylmonomethylethanolamine, PE = phosphatidylethanolamine, PDME = phosphatidyldimethylethanolamine, LPE = lysophosphatidylethanolamine, {{Image200|LBGPEccp:R:R:pCH0001.gif}} <<2426>>/<BR>TLC, plate: Silica gel 60 (Merck, 20 X 20 cm, 0.25 mm thick), solvent: 1st dim. chloroform - methanol - water (65:25:4, v/v), 2nd dim. n-butanol - acetic acid - water (6:2:2, v/v) <<2427>>, | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Latest revision as of 21:00, 14 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | PGP2404 |
| LipidMaps | [1] |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBGPEccp:R:R:p |
| phosphatidyl-N,N-dimethylethanolamine | |
|---|---|
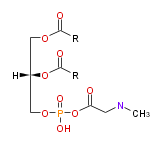
| |
| Structural Information | |
| 1,2-di-acyl-sn-glycero- (3) -phospho-N,N-dimethylethanolamine | |
| |
| Formula | |
| Exact Mass | |
| Average Mass | |
| SMILES | |
| Physicochemical Information | |
| 1,2-distearoyl-L-N,N-dimethylphosphatidylethanolamine, 168-169°C; 1,2-distearoyl-DL-N,N-dimethylphosphatidylethanolamine, 160-162°C; 1,2-dipalmitoyl-L-N,N-dimethylphosphatidylethanolamine, 164-165°C; 1,2-dipalmitoyl-DL-N,N-dimethylphosphatidylethanolamine, 160-161°C <<2437>> | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 2H-NMR spectra of PC, PDME, PMME, and PE fully hydrated with D2O at the indicated temperatures 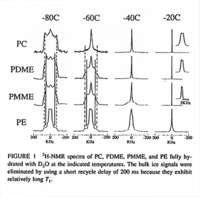 <<2435>>., |
| Other Spectra | |
| Chromatograms | HPLC, μ Bondapak NH2, acetonitril-methanol-water (13:7:1, v/v), elution rate 1.6 ml/min, detection OD203 nm, Peaks: SF = solvent front, PC = phosphatidylcholine, SPH = sphingomyelin, LPC = lysophosphatidylcholine, PMME = phosphatidylmonomethylethanolamine, PE = phosphatidylethanolamine, PDME = phosphatidyldimethylethanolamine, LPE = lysophosphatidylethanolamine, 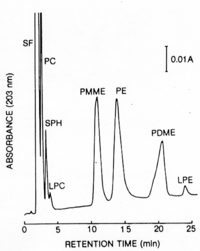 <<2426>>/ TLC, plate: Silica gel 60 (Merck, 20 X 20 cm, 0.25 mm thick), solvent: 1st dim. chloroform - methanol - water (65:25:4, v/v), 2nd dim. n-butanol - acetic acid - water (6:2:2, v/v) <<2427>>, |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|