LBF18303HP06
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8063 |
| LipidMaps | LMFA01040047 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18303HP06 |
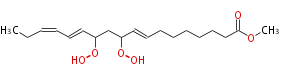
| Methyl-10,12-dihydroperoxy- (8E,13E,15Z) -octadecatrienoic acid | |
|---|---|
| |
| Structural Information | |
| Methyl-10,12-dihydroperoxy- (trans-8,trans-13,cis-15) -octadecatrienoic acid | |
| |
| Formula | C19H32O6 |
| Exact Mass | 356.219888756 |
| Average Mass | 356.45378 |
| SMILES | CCC=CC=CC(OO)CC(OO)C=CCCCCCCC(=O)OC |
| Physicochemical Information | |
| It is produced from 10- or 12-hydroperoxy isomer during oxidation of linoleate by singlet-oxygen Frankel_EN Neff_WE et al. Neff_WE et al.. | |
| It reacts with DNA in the presence of Fe ions and ascorbic acid Fujimoto_K et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after reduction and TMS-derivatization) Neff_WE et al.: m/e=468[M]; 453[M-OCH3]; 437[M-OC H3]; 378[M-HOTMS]; 271[SMTO=CHCH=CH(CH2)6COOCH3]; 183[SMTO=CHCH=CHCH=CHCH2 CH3]; CI-MS(110) m/e=339[M+H-H20]; 323[M-OOH]; 305[323-H20]; 199[CHOCH=CH(CH2)6COOCH3+H] |
| UV Spectra | Conjugated diene: λ max=231-233nm Neff_WE et al. |
| IR Spectra | OOH group: 3700-3140cm-1[bonded], 3520-3510cm-1[free]; conjugated trans, cis diene: 985-979cm-1, 953-935cm-1; isolated trans unsaturation: 968-960cm-1 Neff_WE et al. |
| NMR Spectra | 1H-NMR Neff_WE et al.: C2, 7, 11, 17: 1.8-2.5ppm; C8, 9, 13, 15, 16: 5.3-6.1ppm; C10, 12: 4.49- 4.51ppm; C14: 6.60-6.63ppm; C18: 1.01-1.10ppm; OOH: 8.02-8.05ppm |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|