LBF20406HO20
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR6101 |
| LipidMaps | LMFA03060002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HO20 |
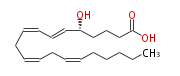
| 5S-Hydroxy- (6E,8Z,11Z,14Z) -eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 5S-Hydroxy- (trans-6,cis-8,cis-11,cis-14) -eicosatetraenoic acid | |
| |
| 5(S)-HETE | |
| Formula | C20H32O3 |
| Exact Mass | 320.23514489 |
| Average Mass | 320.46628 |
| SMILES | C(CC=CCC=CCC=CC=C[C@@H](CCCC(O)=O)O)CCC |
| Physicochemical Information | |
| METHYL ESTER ; [ α ]23 D =+14.0°(C=2.0, BENZENE) | |
| DIETHYL ETHER BorgeatPet al. | |
 | |
| When arachidonic acid is oxygenated by 5-lipoxygenase, 5(S)-hydroperoxy-6,,8,11,14-(E,Z,Z,Z)-eicosatetraenoic acid is produced Ford-Hutchinson_AW et al.. The latter compound is reduced to a corresponding 5(S)-hydroxy acid with whole cells or crude enzyme preparations. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ETHER ; m/e 406(M+), 391, 375, 316, 305, 255, 216, 215, 203, 190, 155, 150, 143, 136, 105, 80, 79 BorgeatPet al. |
| UV Spectra | METHYL ESTER ; λ MeOH max = 235nm ( ε 30,500) BorgeatPet al. |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|