LBF10000BC03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&4-Hexyl Decanoic Acid&& | |Common Name=&&4-Hexyl Decanoic Acid&& | ||
|Boiling Point=147-148°C/0.8mmHg [[Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474|{{RelationTable/GetFirstAuthor|Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474}}]] | |Boiling Point=147-148°C/0.8mmHg [[Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474|{{RelationTable/GetFirstAuthor|Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474}}]] | ||
|Refractive= | |Refractive=eta27/D=1.4450 [[Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474|{{RelationTable/GetFirstAuthor|Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=Diethyl (2-hexyloctyl)malonate was heated with ethanolic KOH, and the product was heated after acidification[[Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474|{{RelationTable/GetFirstAuthor|Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474}}]]. | |Chemical Synthesis=Diethyl (2-hexyloctyl)malonate was heated with ethanolic KOH, and the product was heated after acidification[[Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474|{{RelationTable/GetFirstAuthor|Reference:Asano_M:Yamakawa_T:,J. Pharm. Soc. Jpn.,1950,70,474}}]]. | ||
Revision as of 14:00, 19 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA7140 |
| LipidMaps | LMFA01020172 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF10000BC03 |
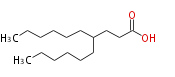
| 4-Hexyl Decanoic Acid | |
|---|---|
| |
| Structural Information | |
| 4-Hexyl Decanoic Acid | |
| |
| Formula | C16H32O2 |
| Exact Mass | 256.240230268 |
| Average Mass | 256.42408 |
| SMILES | CCCCCCC(CCCCCC)CCC(O)=O |
| Physicochemical Information | |
| 147-148°C/0.8mmHg Asano_M et al. | |
| η27/D=1.4450 AsanoMet al. | |
| Diethyl (2-hexyloctyl)malonate was heated with ethanolic KOH, and the product was heated after acidification Asano_M et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|