LBF18000BC11: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|Common Name=&&5-Methyl Octadecanoic Acid&& | |Common Name=&&5-Methyl Octadecanoic Acid&& | ||
|Melting Point=44.5-45°C [[Reference:Cason_J:Winans_WR:,J. Org. Chem.,1950,15,139|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Winans_WR:,J. Org. Chem.,1950,15,139}}]] | |Melting Point=44.5-45°C [[Reference:Cason_J:Winans_WR:,J. Org. Chem.,1950,15,139|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Winans_WR:,J. Org. Chem.,1950,15,139}}]] | ||
|Boiling Point=174°C/0.1mmHg | |Boiling Point=174°C/0.1mmHg<!--7073--> | ||
|UV Spectra=209nm [[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | |UV Spectra=209nm [[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | ||
|IR Spectra=7.76<FONT FACE="Symbol">m</FONT>, 8.25<FONT FACE="Symbol">m</FONT>(CS2) [[Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523|{{RelationTable/GetFirstAuthor|Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523}}]] | |IR Spectra=7.76<FONT FACE="Symbol">m</FONT>, 8.25<FONT FACE="Symbol">m</FONT>(CS2) [[Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523|{{RelationTable/GetFirstAuthor|Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=Diethyl ester of (3-methyl tetradecyl) malonate was heated with ethanolic KOH. After acidification the product was obtained by distillation under deminished pressure. | |Chemical Synthesis=Diethyl ester of (3-methyl tetradecyl) malonate was heated with ethanolic KOH. After acidification the product was obtained by distillation under deminished pressure<!--7073-->. | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity=[[Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523|{{RelationTable/GetFirstAuthor|Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523}}]][[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | |Biological Activity=[[Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523|{{RelationTable/GetFirstAuthor|Reference:Freeman_NK:,J. Am. Chem. Soc.,1952,74,2523}}]][[Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177|{{RelationTable/GetFirstAuthor|Reference:Cason_J:Sumrell_G:,J. Org. Chem.,1951,16,1177}}]] | ||
Revision as of 16:30, 26 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA7145 |
| LipidMaps | LMFA01020177 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18000BC11 |
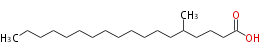
| 5-Methyl Octadecanoic Acid | |
|---|---|
| |
| Structural Information | |
| 5-Methyl Octadecanoic Acid | |
| |
| Formula | C19H38O2 |
| Exact Mass | 298.28718046 |
| Average Mass | 298.50382 |
| SMILES | CCCCCCCCCCCCCC(C)CCCC(O)=O |
| Physicochemical Information | |
| 44.5-45°C Cason_J et al. | |
| 174°C/0.1mmHg | |
| Diethyl ester of (3-methyl tetradecyl) malonate was heated with ethanolic KOH. After acidification the product was obtained by distillation under deminished pressure. | |
| Freeman_NK Cason_J et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | 209nm CasonJet al. |
| IR Spectra | 7.76m, 8.25m(CS2) Freeman_NK |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|