LBF18107PG01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 8: | Line 8: | ||
|SysName=5- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -4-oxopentanoic acid | |SysName=5- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -4-oxopentanoic acid | ||
|Common Name=&&2,3-dinor-6-ketoprostaglandin F_1alpha&&5- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -4-oxopentanoic acid&& | |Common Name=&&2,3-dinor-6-ketoprostaglandin F_1alpha&&5- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -4-oxopentanoic acid&& | ||
|Optical=[<FONT FACE="Symbol">a</FONT>]<SUP><FONT SIZE=-1>2</FONT></SUP><SUP><FONT SIZE=-1>0</FONT></SUP><SUP><FONT SIZE=-1>.</FONT></SUP><SUP><FONT SIZE=-1>2</FONT></SUP><SUB><FONT SIZE=-1>D</FONT></SUB>= +16.9° (C=1.76 CHLOROFORM, measured after 24 hours at 20°C) | |Optical=[<FONT FACE="Symbol">a</FONT>]<SUP><FONT SIZE=-1>2</FONT></SUP><SUP><FONT SIZE=-1>0</FONT></SUP><SUP><FONT SIZE=-1>.</FONT></SUP><SUP><FONT SIZE=-1>2</FONT></SUP><SUB><FONT SIZE=-1>D</FONT></SUB>= +16.9° (C=1.76 CHLOROFORM, measured after 24 hours at 20°C) <!--1060--> | ||
|Solubility=METHANOL [[Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115}}]] | |Solubility=METHANOL [[Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115}}]] | ||
|Mass Spectra=METHOXIME TRI-TMS ETHER METHYL ESTER ; m/e 601(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 586, 570, 530, 511, 496, 480, 440, 421, 390, 350, 300, 294, 263, 217, 205, 191, 73 [[Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115}}]] | |Mass Spectra=METHOXIME TRI-TMS ETHER METHYL ESTER ; m/e 601(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 586, 570, 530, 511, 496, 480, 440, 421, 390, 350, 300, 294, 263, 217, 205, 191, 73 [[Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115|{{RelationTable/GetFirstAuthor|Reference:Pace-Asciak_CR:Carrara_MC:Domazet_Z:,Biochem. Biophys. Res. Commun.,1977,78,115}}]] | ||
|Source=When prostaglandin I2 or its non-enzymatic degradation product (6-keto-prostaglandin F1<FONT FACE="Symbol">a</FONT>) was infused into man, a major urinary metbolite was 2,3-dinor-6-keto-prostaglandin F1<FONT FACE="Symbol">a</FONT>, a <FONT FACE="Symbol">b</FONT>-oxidation product [[Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69}}]]. | |Source=When prostaglandin I2 or its non-enzymatic degradation product (6-keto-prostaglandin F1<FONT FACE="Symbol">a</FONT>) was infused into man, a major urinary metbolite was 2,3-dinor-6-keto-prostaglandin F1<FONT FACE="Symbol">a</FONT>, a <FONT FACE="Symbol">b</FONT>-oxidation product [[Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69}}]]. | ||
|Chemical Synthesis= {{Image200|LBF18107PG01FT0001.gif}} | |Chemical Synthesis=<!--1060--> {{Image200|LBF18107PG01FT0001.gif}} | ||
|Metabolism= | |Metabolism= | ||
|Symbol=2,3-DINOR-6-KETO-PGF1<FONT FACE="Symbol">a</FONT> | |Symbol=2,3-DINOR-6-KETO-PGF1<FONT FACE="Symbol">a</FONT> | ||
Revision as of 16:30, 26 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1821 |
| LipidMaps | LMFA03010089 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18107PG01 |
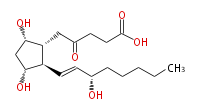
| 2,3-dinor-6-ketoprostaglandin F_1α | |
|---|---|
| |
| Structural Information | |
| 5- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -4-oxopentanoic acid | |
| |
| 2,3-DINOR-6-KETO-PGF1a | |
| Formula | C18H30O6 |
| Exact Mass | 342.204238692 |
| Average Mass | 342.42719999999997 |
| SMILES | [C@@H]([C@@H](CC(=O)CCC(O)=O)1)(C=C[C@H](O)CCCCC)[C@H](O)C[C@@H]1O |
| Physicochemical Information | |
| [a]20.2D= +16.9° (C=1.76 CHLOROFORM, measured after 24 hours at 20°C) | |
| METHANOL Pace-Asciak_CR et al. | |
| When prostaglandin I2 or its non-enzymatic degradation product (6-keto-prostaglandin F1a) was infused into man, a major urinary metbolite was 2,3-dinor-6-keto-prostaglandin F1a, a b-oxidation product Needleman_P et al.. | |
| Spectral Information | |
| Mass Spectra | METHOXIME TRI-TMS ETHER METHYL ESTER ; m/e 601(M+), 586, 570, 530, 511, 496, 480, 440, 421, 390, 350, 300, 294, 263, 217, 205, 191, 73 Pace-Asciak_CR et al. |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|