LBF18207HO03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|LipidMaps=LMFA01050125 | |LipidMaps=LMFA01050125 | ||
|SysName=13-Hydroxy-9,11-Octadecadienoic Acid/13-Hydroxy-9,11-Octadecadienoate | |SysName=13-Hydroxy-9,11-Octadecadienoic Acid/13-Hydroxy-9,11-Octadecadienoate | ||
|Common Name=&&13-Hydroxy-9,11-Octadecadienoic Acid | |Common Name=&&13-Hydroxy-9,11-Octadecadienoic Acid&&13-Hydroxy-9,11-Octadecadienoate&& | ||
|Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]][[Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109|{{RelationTable/GetFirstAuthor|Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109}}]]: m/e=382[M], 367[M-CH3], 351[M-OCH3], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]], GC-EI-MS(after methanolysis and hydrogenation) [[Reference:Christophersen_BO:,Biochim. Biophys. Acta,1968,164,35|{{RelationTable/GetFirstAuthor|Reference:Christophersen_BO:,Biochim. Biophys. Acta,1968,164,35}}]] | |Mass Spectra=GC-EI-MS(after methanolysis and trimethylsilylation)[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]][[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31|{{RelationTable/GetFirstAuthor|Reference:Kleiman_R:Spencer_GF:,J. Am. Oil Chem. Soc.,1973,50,31}}]][[Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908|{{RelationTable/GetFirstAuthor|Reference:Frankel_EN:Neff_WE:Rohwedder_WK:Khambay_BP:Garwood_RF:Weedon_BC:,Lipids,1977,12,908}}]][[Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109|{{RelationTable/GetFirstAuthor|Reference:Lundberg_WO:Chipault_JR:Hendrickson_NJ:,J. Am. Oil Chem. Soc.,1949,26,109}}]]: m/e=382[M], 367[M-CH3], 351[M-OCH3], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation)[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]][[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]], GC-EI-MS(after methanolysis and hydrogenation) [[Reference:Christophersen_BO:,Biochim. Biophys. Acta,1968,164,35|{{RelationTable/GetFirstAuthor|Reference:Christophersen_BO:,Biochim. Biophys. Acta,1968,164,35}}]] | ||
|UV Spectra=<FONT FACE="Symbol">l</FONT>EtOH/max=234nm(conjugated diene)[[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | |UV Spectra=<FONT FACE="Symbol">l</FONT>EtOH/max=234nm(conjugated diene)[[Reference:Hamberg_M:,Lipids,1975,10,87|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:,Lipids,1975,10,87}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | ||
Revision as of 00:02, 20 December 2008
| IDs and Links | |
|---|---|
| LipidBank | DFA8023 |
| LipidMaps | LMFA01050125 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18207HO03 |
| 13-Hydroxy-9,11-Octadecadienoic Acid | |
|---|---|
| |
| Structural Information | |
| 13-Hydroxy-9,11-Octadecadienoic Acid/13-Hydroxy-9,11-Octadecadienoate | |
| |
| Formula | C18H32O3 |
| Exact Mass | 296.23514489 |
| Average Mass | 296.44488 |
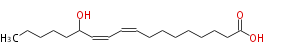
| SMILES | CCCCCC(O)C=CC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) Gardner_HW et al. StreckertGet al. KleimanRet al. Frankel_EN et al. Lundberg_WO et al.: m/e=382[M], 367[M-CH3], 351[M-OCH3], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) StreckertGet al. HambergM, GC-EI-MS(after methanolysis and hydrogenation) Christophersen_BO |
| UV Spectra | lEtOH/max=234nm(conjugated diene) HambergM Sessa_DJ et al. |
| IR Spectra | Methyl ester: trans, trans isomer: trans, trans conjugated dinen(985cm-1), free OH(3600cm-1), bonded OH(3695-3318cm-1); cis, trans isomer: cis, trans conjugated diene(990, 968cm-1), olefinic(3005cm-1), free OH(3600cm-1), bonded OH(3700-3160cm |
| NMR Spectra | 1H-NMR(methyl ester): trans, trans olefinic protons(5.41ppm), cis,trans olefinic protons(5.91ppm), C13(4.15-4.20ppm), C8(2.07-2.10ppm) Neff_WE et al. Sessa_DJ et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|