LBF18207HO03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
|IR Spectra=Methyl ester: trans, trans isomer: trans, trans conjugated dinen(985cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), free OH(3600cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), bonded OH(3695-3318cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>); cis, trans isomer: cis, trans conjugated diene(990, 968cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), olefinic(3005cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), free OH(3600cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), bonded OH(3700-3160cm | |IR Spectra=Methyl ester: trans, trans isomer: trans, trans conjugated dinen(985cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), free OH(3600cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), bonded OH(3695-3318cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>); cis, trans isomer: cis, trans conjugated diene(990, 968cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), olefinic(3005cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), free OH(3600cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP>), bonded OH(3700-3160cm | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(methyl ester): trans, trans olefinic protons(5.41ppm), cis,trans olefinic protons(5.91ppm), C13(4.15-4.20ppm), C8(2.07-2.10ppm) [[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(methyl ester): trans, trans olefinic protons(5.41ppm), cis,trans olefinic protons(5.91ppm), C13(4.15-4.20ppm), C8(2.07-2.10ppm) [[Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415|{{RelationTable/GetFirstAuthor|Reference:Neff_WE:Frankel_EN:Scholfield_CR:Wesleder_D:,Lipids,1978,13,415}}]][[Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613|{{RelationTable/GetFirstAuthor|Reference:Sessa_DJ:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1977,12,613}}]] | ||
|Source=Minor degradation products of linoleate hydroperoxide in the presence of Fe(III)-cystein[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]] | |Source=Minor degradation products of linoleate hydroperoxide in the presence of Fe(III)-cystein[[Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696|{{RelationTable/GetFirstAuthor|Reference:Gardner_HW:Kleiman_R:Weisleder_D:,Lipids,1974,9,696}}]]. A degradation product of hydroperoxymethyllinoleate in the presence of di-t-butyl diperoxyoxalate or Co(II)[[Reference:Yamamoto_Y:Saeki_N:Haga_S:Niki_E:_Kamiya_Y:,Bull. Chem. Soc. Jpn.,1984,57,3177|{{RelationTable/GetFirstAuthor|Reference:Yamamoto_Y:Saeki_N:Haga_S:Niki_E:_Kamiya_Y:,Bull. Chem. Soc. Jpn.,1984,57,3177}}]]. Reaction products bstween hydroperoxylinoleate and soy bean lipoxygenase[[Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847|{{RelationTable/GetFirstAuthor|Reference:Streckert_G:Stan_HJ:,Lipids,1975,10,847}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
Revision as of 11:55, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8023 |
| LipidMaps | LMFA01050125 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18207HO03 |
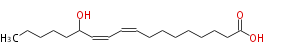
| 13-Hydroxy-9,11-Octadecadienoic Acid | |
|---|---|
| |
| Structural Information | |
| 13-Hydroxy-9,11-Octadecadienoic Acid/13-Hydroxy-9,11-Octadecadienoate | |
| |
| Formula | C18H32O3 |
| Exact Mass | 296.23514489 |
| Average Mass | 296.44488 |
| SMILES | CCCCCC(O)C=CC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| Minor degradation products of linoleate hydroperoxide in the presence of Fe(III)-cystein Gardner_HW et al.. A degradation product of hydroperoxymethyllinoleate in the presence of di-t-butyl diperoxyoxalate or Co(II) Yamamoto_Y et al.. Reaction products bstween hydroperoxylinoleate and soy bean lipoxygenase Streckert_G et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) Gardner_HW et al. StreckertGet al. KleimanRet al. Frankel_EN et al. Lundberg_WO et al.: m/e=382[M], 367[M-CH3], 351[M-OCH3], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) StreckertGet al. HambergM, GC-EI-MS(after methanolysis and hydrogenation) Christophersen_BO |
| UV Spectra | lEtOH/max=234nm(conjugated diene) HambergM Sessa_DJ et al. |
| IR Spectra | Methyl ester: trans, trans isomer: trans, trans conjugated dinen(985cm-1), free OH(3600cm-1), bonded OH(3695-3318cm-1); cis, trans isomer: cis, trans conjugated diene(990, 968cm-1), olefinic(3005cm-1), free OH(3600cm-1), bonded OH(3700-3160cm |
| NMR Spectra | 1H-NMR(methyl ester): trans, trans olefinic protons(5.41ppm), cis,trans olefinic protons(5.91ppm), C13(4.15-4.20ppm), C8(2.07-2.10ppm) Neff_WE et al. Sessa_DJ et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|