LBF18207HO03: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
|Metabolism= | |Metabolism= | ||
|Biological Activity=Trans, trans configuration showed a slightly lower toxicity than linoleate monohydroxyperoxide[[Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21}}]]. | |Biological Activity=Trans, trans configuration showed a slightly lower toxicity than linoleate monohydroxyperoxide<!--8045-->[[Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21|{{RelationTable/GetFirstAuthor|Reference:Fujimoto_K:,Fragrance J. (in Japanese),1986,76,21}}]]. | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 16:30, 26 January 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8023 |
| LipidMaps | LMFA01050125 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF18207HO03 |
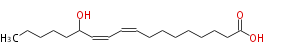
| 13-Hydroxy-9,11-Octadecadienoic Acid | |
|---|---|
| |
| Structural Information | |
| 13-Hydroxy-9,11-Octadecadienoic Acid/13-Hydroxy-9,11-Octadecadienoate | |
| |
| Formula | C18H32O3 |
| Exact Mass | 296.23514489 |
| Average Mass | 296.44488 |
| SMILES | CCCCCC(O)C=CC=CCCCCCCCC(O)=O |
| Physicochemical Information | |
| Minor degradation products of linoleate hydroperoxide in the presence of Fe(III)-cystein Gardner_HW et al.. A degradation product of hydroperoxymethyllinoleate in the presence of di-t-butyl diperoxyoxalate or Co(II) Yamamoto_Y et al.. Reaction products bstween hydroperoxylinoleate and soy bean lipoxygenase Streckert_G et al.. | |
| Trans, trans configuration showed a slightly lower toxicity than linoleate monohydroxyperoxide Fujimoto_K . | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(after methanolysis and trimethylsilylation) Gardner_HW et al. StreckertGet al. KleimanRet al. Frankel_EN et al. Lundberg_WO et al.: m/e=382[M], 367[M-CH3], 351[M-OCH3], 311[M-(CH2)4CH3], 225[M-(CH2)7COOCH3], GC-EI-MS(after methanolysis, hydrogenation and trimethylsilylation) StreckertGet al. HambergM, GC-EI-MS(after methanolysis and hydrogenation) Christophersen_BO |
| UV Spectra | lEtOH/max=234nm(conjugated diene) HambergM Sessa_DJ et al. |
| IR Spectra | Methyl ester: trans, trans isomer: trans, trans conjugated dinen(985cm-1), free OH(3600cm-1), bonded OH(3695-3318cm-1); cis, trans isomer: cis, trans conjugated diene(990, 968cm-1), olefinic(3005cm-1), free OH(3600cm-1), bonded OH(3700-3160cm |
| NMR Spectra | 1H-NMR(methyl ester): trans, trans olefinic protons(5.41ppm), cis,trans olefinic protons(5.91ppm), C13(4.15-4.20ppm), C8(2.07-2.10ppm) Neff_WE et al. Sessa_DJ et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|