LBF20107PG18: Difference between revisions
No edit summary |
No edit summary |
||
| Line 13: | Line 13: | ||
|IR Spectra=NEAT: <FONT FACE="Symbol">n</FONT> 3400, 1715, 1245, 1045, 975, 915, 875, 845, 800, 730 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | |IR Spectra=NEAT: <FONT FACE="Symbol">n</FONT> 3400, 1715, 1245, 1045, 975, 915, 875, 845, 800, 730 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(ACETONE-D<SUB><FONT SIZE=-1>6</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 6.1-5.4(bs, 4H), 5.5-5.2(m, 2H), 4.7-3.5(m, 3H), 2.5-1.1 (m, 22H), 0.86(t, 3H) [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(ACETONE-D<SUB><FONT SIZE=-1>6</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 6.1-5.4(bs, 4H), 5.5-5.2(m, 2H), 4.7-3.5(m, 3H), 2.5-1.1 (m, 22H), 0.86(t, 3H) [[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]] | ||
|Source=When prostaglandin I2 is produced in animal tissues, it is unstable in aqueous solution, especially at acidic pH, and readily decomposed to 6-keto-prostaglandin F1<FONT FACE="Symbol">a</FONT> [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]];>. Therefore, 6-keto-prostaglandin F1<FONT FACE="Symbol">a</FONT> is detected where prostaglandin I2 is produced. | |||
|Chemical Synthesis=[[Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813|{{RelationTable/GetFirstAuthor|Reference:Tanaka_T:Hazato_A:Bannai_K:Okamura_N:Sugiura_S:Manabe_K:Toru_T:Kurozumi_S:Suzuki_M:Kawagishi_T_et_al:,Tetrahedron,1987,43,813}}]];> {{Image200|XPR1811FT0001.gif}} | |||
|Metabolism=6-Keto-prostaglandin F1<FONT FACE="Symbol">a</FONT> is subjected to <FONT FACE="Symbol">b</FONT>-oxidation, and converted to 2,3-dinor-6-keto-prostaglandin F1<FONT FACE="Symbol">a</FONT> which appears in urine as a major metabolite [[Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69}}]];>. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 22:00, 24 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1811 |
| LipidMaps | LMFA03010001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20107PG18 |
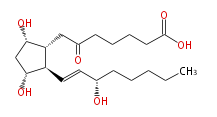
| 6-KETOPROSTAGLANDIN F_1α | |
|---|---|
| |
| Structural Information | |
| 7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) cyclopentan-1 (R) -yl ] -6-oxoheptanoic acid | |
| |
| Formula | C20H34O6 |
| Exact Mass | 370.23553882 |
| Average Mass | 370.48036 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@@H](CC(=O)CCCCC(O)=O)1)[C@@H](C[C@@H]1O)O)CC |
| Physicochemical Information | |
| DIETHYL ETHER Pace-AsciakCMETHANOL, ACETONE, ETHYL ACETATE TanakaTet al. | |
| When prostaglandin I2 is produced in animal tissues, it is unstable in aqueous solution, especially at acidic pH, and readily decomposed to 6-keto-prostaglandin F1a Moncada_S et al.;>. Therefore, 6-keto-prostaglandin F1a is detected where prostaglandin I2 is produced. | |
|
Tanaka_T et al.;> File:XPR1811FT0001.gif | |
| 6-Keto-prostaglandin F1a is subjected to b-oxidation, and converted to 2,3-dinor-6-keto-prostaglandin F1a which appears in urine as a major metabolite Needleman_P et al.;>. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; 366(M+-18), 348, 335, 330, 323, 319, 279, 265, 223, 196, 195, 164, 143, 121, 111, 99, 95, 71 TanakaTet al.. DIRECT EXPOSURE AMMONIA CI POSITIVE : 370, 353, 244, 163, 153, 136. NEGATIVE : 368, 351, 334, 316, 225, 219, 166, 135, 127 Cepa_SR et al. |
| UV Spectra | |
| IR Spectra | NEAT: n 3400, 1715, 1245, 1045, 975, 915, 875, 845, 800, 730 cm-1 TanakaTet al. |
| NMR Spectra | 1H-NMR(ACETONE-D6) : d 6.1-5.4(bs, 4H), 5.5-5.2(m, 2H), 4.7-3.5(m, 3H), 2.5-1.1 (m, 22H), 0.86(t, 3H) TanakaTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|