LBF20207PG01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
|Solubility=ETHANOL, CHLOROFORM [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]], METHANOL [[Reference:Andersen_NH:,J. Lipid Res.,1969,10,320|{{RelationTable/GetFirstAuthor|Reference:Andersen_NH:,J. Lipid Res.,1969,10,320}}]] | |Solubility=ETHANOL, CHLOROFORM [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]], METHANOL [[Reference:Andersen_NH:,J. Lipid Res.,1969,10,320|{{RelationTable/GetFirstAuthor|Reference:Andersen_NH:,J. Lipid Res.,1969,10,320}}]] | ||
|Mass Spectra=m/e, 336(M^+ ), 318(M-18), 300(M-36), 265(M-71), 247, 219, 190 [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | |Mass Spectra=m/e, 336(M^+ ), 318(M-18), 300(M-36), 265(M-71), 247, 219, 190 [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|UV Spectra= lambda ^ | |UV Spectra= lambda ^{EtOH}_{max} = 217nm( epsilon 11,650) [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|IR Spectra=NUJOL : nu 3420, 2740, 2700, 2660, 2600, 1715, 1700, 1585, 1275, 1200,1180, 1020, 720cm^- | |IR Spectra=NUJOL : nu 3420, 2740, 2700, 2660, 2600, 1715, 1700, 1585, 1275, 1200,1180, 1020, 720cm^{-1} [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|NMR Spectra=^1 H-NMR(CDCl_3 , TMS) : delta , 7.52(dd, 1H), 6.17(dd, 1H), 5.6(m, 2H), 4.1(m, 1H, 15-CH), 3.25(12H) [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | |NMR Spectra=^1 H-NMR(CDCl_3 , TMS) : delta , 7.52(dd, 1H), 6.17(dd, 1H), 5.6(m, 2H), 4.1(m, 1H, 15-CH), 3.25(12H) [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|NOTE Spectra=ORD(H_2 O, C=0.0040g/100ml), 256(+35, 000°), 220(-43,000°) [[Reference:Andersen_NH:,J. Lipid Res.,1969,10,320|{{RelationTable/GetFirstAuthor|Reference:Andersen_NH:,J. Lipid Res.,1969,10,320}}]] | |NOTE Spectra=ORD(H_2 O, C=0.0040g/100ml), 256(+35, 000°), 220(-43,000°) [[Reference:Andersen_NH:,J. Lipid Res.,1969,10,320|{{RelationTable/GetFirstAuthor|Reference:Andersen_NH:,J. Lipid Res.,1969,10,320}}]] | ||
Revision as of 10:00, 25 February 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1000 |
| LipidMaps | LMFA03010005 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG01 |
| PROSTAGLANDIN A1 | |
|---|---|
| |
| Structural Information | |
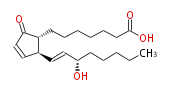
| 7- [ 2 (R) - (3 (S) -Hydroxy-1 (E) -octenyl) -5-oxo-3-cyclopenten-1 (R) -yl ] heptanoic acid / (8R,12S,13E,15S) -15-Hydroxy-9-oxo-10,13-prostadienoic acid | |
| |
| PGA1 | |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CCCCCCC(O)=O)C=CC(=O)1)CC |
| Physicochemical Information | |
| 42-44°C Pike_J_E et al. | |
| ORD (H2O, C=0.0040g/ml) : 256(+35.000°), 220(-43.000°) Andersen_NH | |
| ETHANOL, CHLOROFORM Pike_JEet al., METHANOL Andersen_NH | |
| Prostaglandin A1 is found in human seminal plasma Bergstrom_S . | |
|
Pike_J_E et al. | |
| Prostaglandin A1 relaxes uterine muscle and shows hypotensive effects on rat and dog Bergstrom_S et al.. | |
| Spectral Information | |
| Mass Spectra | m/e, 336(M+), 318(M-18), 300(M-36), 265(M-71), 247, 219, 190 Pike_JEet al. |
| UV Spectra | λ EtOH max = 217nm( ε 11,650) Pike_JEet al. |
| IR Spectra | NUJOL : ν 3420, 2740, 2700, 2660, 2600, 1715, 1700, 1585, 1275, 1200,1180, 1020, 720cm-1 Pike_JEet al. |
| NMR Spectra | 1H-NMR(CDCl3, TMS) : δ , 7.52(dd, 1H), 6.17(dd, 1H), 5.6(m, 2H), 4.1(m, 1H, 15-CH), 3.25(12H) Pike_JEet al. |
| Other Spectra | ORD(H2O, C=0.0040g/100ml), 256(+35, 000°), 220(-43,000°) Andersen_NH |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|