LBF20207PG25: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
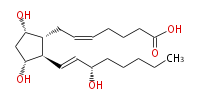
|SysName=7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenylcyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |SysName=7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenylcyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | ||
|Common Name=&&PROSTAGLANDIN F2alpha&& | |Common Name=&&PROSTAGLANDIN F2alpha&& | ||
|Melting Point=25-35°C | |Melting Point=25-35°C [[Reference:Bundy_GL:Schneider_WP:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2123|{{RelationTable/GetFirstAuthor|Reference:Bundy_GL:Schneider_WP:Lincoln_FH:Pike_JE:,J. Am. Chem. Soc.,1972,94,2123}}]] | ||
|Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>25</sup>=23.8 °(C=1,THF) | |Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>25</sup>=23.8 °(C=1,THF) [[Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Schaaf_TK:Huber_W:Koelliker_U:Weinshenker_NM:,J. Am. Chem. Soc.,1970,92,397}}]] | ||
|Solubility= ETHYL ACETATE, ACETONE, DIETHYLETHER | |Solubility= ETHYL ACETATE, ACETONE, DIETHYLETHER [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]]. STABILITIES: to be stable under neutral and basic conditions [[Reference:Karim_SM:Devlin_J:Hillier_K:,Eur. J. Pharmacol.,1968,4,416|{{RelationTable/GetFirstAuthor|Reference:Karim_SM:Devlin_J:Hillier_K:,Eur. J. Pharmacol.,1968,4,416}}]] | ||
|Mass Spectra=354(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 336, 318, 292, 274, 264(100), 247, 229, 191, 177, 165, 137, 99, 81, 67 | |Mass Spectra=354(M<SUP><FONT SIZE=-1>+</FONT></SUP>), 336, 318, 292, 274, 264(100), 247, 229, 191, 177, 165, 137, 99, 81, 67 [[Reference:Horvath_G:,Biomed. Mass Spectrom.,1976,3,127|{{RelationTable/GetFirstAuthor|Reference:Horvath_G:,Biomed. Mass Spectrom.,1976,3,127}}]] | ||
|IR Spectra=NEAT : 3320, 2640, 1710, 1295, 1260, 1245, 1120, 1080, 1055, 1025, 975cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=NEAT : 3320, 2640, 1710, 1295, 1260, 1245, 1120, 1080, 1055, 1025, 975cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(d<SUB><FONT SIZE=-1>6</FONT></SUB>-ACETONE) : <FONT FACE="Symbol">d</FONT> 5.48(m, 4H), 4.05(m, 3H), 0.9(t, 3H, 20-CH3) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(d<SUB><FONT SIZE=-1>6</FONT></SUB>-ACETONE) : <FONT FACE="Symbol">d</FONT> 5.48(m, 4H), 4.05(m, 3H), 0.9(t, 3H, 20-CH3) [[Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552|{{RelationTable/GetFirstAuthor|Reference:Pike_J_E:Lincoln_FH:Schneider_WP:,J. Org. Chem.,1969,34,3552}}]]. <SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>3</FONT></SUP>C-NMR : 176.6(C1), 135.0(C14), 132.8(C5), 129.1(C13 or C6), 128.9(C6 or C13), 77.2(C11), 72.9(C15), 71.8(C9), 55.0(C12), 49.9(C8), 42,6(C10), 36.8(C16), 33.2(C2), 31,5(C18), 26.3(C4), 25.1(C7), 25.1(C17), 24.5 [[Reference:Lukacs_G:Piriou_F:Gero_SD:,Tetrah. Lett.,1973,,515|{{RelationTable/GetFirstAuthor|Reference:Lukacs_G:Piriou_F:Gero_SD:,Tetrah. Lett.,1973,,515}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1501 |
| LipidMaps | LMFA03010002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG25 |
| PROSTAGLANDIN F2α | |
|---|---|
| |
| Structural Information | |
| 7- [ 3 (R) ,5 (S) -Dihydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenylcyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |
| |
| Formula | C20H34O5 |
| Exact Mass | 354.240624198 |
| Average Mass | 354.48096000000004 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)[C@@H](C[C@@H]1O)O)CC |
| Physicochemical Information | |
| 25-35°C Bundy_GL et al. | |
| ETHYL ACETATE, ACETONE, DIETHYLETHER Pike_JEet al.. STABILITIES: to be stable under neutral and basic conditions Karim_SM et al. | |
| Spectral Information | |
| Mass Spectra | 354(M+), 336, 318, 292, 274, 264(100), 247, 229, 191, 177, 165, 137, 99, 81, 67 HorvathG |
| UV Spectra | |
| IR Spectra | NEAT : 3320, 2640, 1710, 1295, 1260, 1245, 1120, 1080, 1055, 1025, 975cm-1 Pike_JEet al. |
| NMR Spectra | 1H-NMR(d6-ACETONE) : d 5.48(m, 4H), 4.05(m, 3H), 0.9(t, 3H, 20-CH3) Pike_JEet al.. 13C-NMR : 176.6(C1), 135.0(C14), 132.8(C5), 129.1(C13 or C6), 128.9(C6 or C13), 77.2(C11), 72.9(C15), 71.8(C9), 55.0(C12), 49.9(C8), 42,6(C10), 36.8(C16), 33.2(C2), 31,5(C18), 26.3(C4), 25.1(C7), 25.1(C17), 24.5 LukacsGet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|