LBF20207PG51: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|SysName=5 (Z) - [ 7 (R) -Hydroxy-6 (R) - (3 (S) -hydroxyocten-1 (E) -yl) -1 (S) ,5 (R) -2-oxabicyclo [ 3.3.0 ] oct-3-ylidenpentanoic acid | |SysName=5 (Z) - [ 7 (R) -Hydroxy-6 (R) - (3 (S) -hydroxyocten-1 (E) -yl) -1 (S) ,5 (R) -2-oxabicyclo [ 3.3.0 ] oct-3-ylidenpentanoic acid | ||
|Common Name=&&PROSTAGLANDIN I2&& | |Common Name=&&PROSTAGLANDIN I2&& | ||
|Reflactive=[<FONT FACE="Symbol">a</FONT>]<SUB><FONT SIZE=-1>D</FONT></SUB>=78°(C=0.8820, CHCl3) | |Reflactive=[<FONT FACE="Symbol">a</FONT>]<SUB><FONT SIZE=-1>D</FONT></SUB>=78°(C=0.8820, CHCl3) [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | ||
|Mass Spectra=11,15-BIS(TRIMETHYLSILYL) ETHER METHYL ESTER ; 495(M<SUP><FONT SIZE=-1>+</FONT></SUP>-CH<SUB><FONT SIZE=-1>3</FONT></SUB>), 479, 439, 423, 349, 327, 323, 315, 313, 199, 173 | |Mass Spectra=11,15-BIS(TRIMETHYLSILYL) ETHER METHYL ESTER ; 495(M<SUP><FONT SIZE=-1>+</FONT></SUP>-CH<SUB><FONT SIZE=-1>3</FONT></SUB>), 479, 439, 423, 349, 327, 323, 315, 313, 199, 173 [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | ||
|IR Spectra=METHYL ESTER ; LIQUID MELT <FONT FACE="Symbol">n</FONT> 3370, 1740, 1695cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=METHYL ESTER ; LIQUID MELT <FONT FACE="Symbol">n</FONT> 3370, 1740, 1695cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>-H-NMR(D<SUB><FONT SIZE=-1>2</FONT></SUB>O, GLYCINE BUFFER, pH10.4) : <FONT FACE="Symbol">d</FONT> 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH<SUB><FONT SIZE=-1>2</FONT></SUB>) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>-H-NMR(D<SUB><FONT SIZE=-1>2</FONT></SUB>O, GLYCINE BUFFER, pH10.4) : <FONT FACE="Symbol">d</FONT> 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH<SUB><FONT SIZE=-1>2</FONT></SUB>) [[Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1801 |
| LipidMaps | LMFA03010087 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG51 |
| PROSTAGLANDIN I2 | |
|---|---|
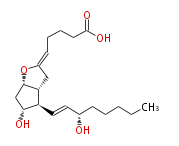
| |
| Structural Information | |
| 5 (Z) - [ 7 (R) -Hydroxy-6 (R) - (3 (S) -hydroxyocten-1 (E) -yl) -1 (S) ,5 (R) -2-oxabicyclo [ 3.3.0 ] oct-3-ylidenpentanoic acid | |
| |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]12)[C@H](O)C[C@H](OC(C2)=CCCCC(O)=O)1)CC |
| Physicochemical Information | |
| Spectral Information | |
| Mass Spectra | 11,15-BIS(TRIMETHYLSILYL) ETHER METHYL ESTER ; 495(M+-CH3), 479, 439, 423, 349, 327, 323, 315, 313, 199, 173 Johnson_RA et al. |
| UV Spectra | |
| IR Spectra | METHYL ESTER ; LIQUID MELT n 3370, 1740, 1695cm-1 Johnson_RA et al. |
| NMR Spectra | 1-H-NMR(D2O, GLYCINE BUFFER, pH10.4) : d 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH2) KotovychGet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|