LBF20207PG51: Difference between revisions
m (LBF20407PG01 moved to LBF20207PG51) |
m (LBF20407PG01 moved to LBF20207PG51) |
||
| Line 12: | Line 12: | ||
|IR Spectra=METHYL ESTER ; LIQUID MELT <FONT FACE="Symbol">n</FONT> 3370, 1740, 1695cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | |IR Spectra=METHYL ESTER ; LIQUID MELT <FONT FACE="Symbol">n</FONT> 3370, 1740, 1695cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>-H-NMR(D<SUB><FONT SIZE=-1>2</FONT></SUB>O, GLYCINE BUFFER, pH10.4) : <FONT FACE="Symbol">d</FONT> 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH<SUB><FONT SIZE=-1>2</FONT></SUB>) [[Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>-H-NMR(D<SUB><FONT SIZE=-1>2</FONT></SUB>O, GLYCINE BUFFER, pH10.4) : <FONT FACE="Symbol">d</FONT> 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH<SUB><FONT SIZE=-1>2</FONT></SUB>) [[Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974|{{RelationTable/GetFirstAuthor|Reference:Kotovych_G:Aarts_GHM:Takashima_TT:Bigam_G:,Can. J. Chem.,1980,58,974}}]] | ||
|Source=In most animal tissues prostanoids are synthesized enzymatically de novo upon physiological and pathological stimulations, and this is also the case of prostaglandin I2. Prostaglandin I2 is produced in blood vessels, lung and other tissues [[Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293|{{RelationTable/GetFirstAuthor|Reference:Moncada_S:Vane_JR:,Pharmacol. Rev.,1978,30,293}}]];>. | |||
|Chemical Synthesis=[[Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690|{{RelationTable/GetFirstAuthor|Reference:Johnson_RA:Lincoln_FH:Nidy_EG:Schneider_WP:Thompson_JL:Axen_U:,J. Am. Chem. Soc.,1978,100,7690}}]];> {{Image200|XPR1801FT0001.gif}} | |||
|Metabolism=Prostaaglandin I2 is produced by isomerization of 9,11-endoperoxide of prostaglandin H2 by the catalysis of prostaglandin I synthaase [[Reference:Tanabe_T:Ullrich_V:,J. Lipid Mediat. Cell Signal.,1995,12,243|{{RelationTable/GetFirstAuthor|Reference:Tanabe_T:Ullrich_V:,J. Lipid Mediat. Cell Signal.,1995,12,243}}]];>. Prostaglandin I2 is unstable and decomposes readily to 6-keto-prostaglandin F2<FONT FACE="Symbol">a</FONT>. Its 2,3-dinor derivative is a major urinary metabolite [[Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69|{{RelationTable/GetFirstAuthor|Reference:Needleman_P:Turk_J:Jakschik_BA:Morrison_AR:Lefkowith_JB:,Annu. Rev. Biochem.,1986,55,69}}]];>. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 22:00, 24 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR1801 |
| LipidMaps | LMFA03010087 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG51 |
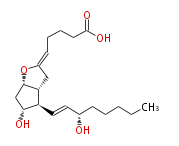
| PROSTAGLANDIN I2 | |
|---|---|
| |
| Structural Information | |
| 5 (Z) - [ 7 (R) -Hydroxy-6 (R) - (3 (S) -hydroxyocten-1 (E) -yl) -1 (S) ,5 (R) -2-oxabicyclo [ 3.3.0 ] oct-3-ylidenpentanoic acid | |
| |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]12)[C@H](O)C[C@H](OC(C2)=CCCCC(O)=O)1)CC |
| Physicochemical Information | |
| In most animal tissues prostanoids are synthesized enzymatically de novo upon physiological and pathological stimulations, and this is also the case of prostaglandin I2. Prostaglandin I2 is produced in blood vessels, lung and other tissues Moncada_S et al.;>. | |
|
Johnson_RA et al.;> File:XPR1801FT0001.gif | |
| Prostaaglandin I2 is produced by isomerization of 9,11-endoperoxide of prostaglandin H2 by the catalysis of prostaglandin I synthaase Tanabe_T et al.;>. Prostaglandin I2 is unstable and decomposes readily to 6-keto-prostaglandin F2a. Its 2,3-dinor derivative is a major urinary metabolite Needleman_P et al.;>. | |
| Spectral Information | |
| Mass Spectra | 11,15-BIS(TRIMETHYLSILYL) ETHER METHYL ESTER ; 495(M+-CH3), 479, 439, 423, 349, 327, 323, 315, 313, 199, 173 Johnson_RA et al. |
| UV Spectra | |
| IR Spectra | METHYL ESTER ; LIQUID MELT n 3370, 1740, 1695cm-1 Johnson_RA et al. |
| NMR Spectra | 1-H-NMR(D2O, GLYCINE BUFFER, pH10.4) : d 5.60(m, 2H, 13,14-CH), 4.66(m, 1H, 9-CH), 4.39(t, 1H, 5-CH), 4.15(q, 1H, 15-CH), 3.97(q, 1H, 11-CH), 2.20(t, 2H, 2-CH2) KotovychGet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|