LBF20406HP10: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|LipidBank=DFA8082 | |LipidBank=DFA8082 | ||
|LipidMaps=LMFA03060041 | |LipidMaps=LMFA03060041 | ||
|SysName=11-Hydroperoxy- | |SysName=11-Hydroperoxy- 5,8,12,14 -eicosatetraenoic acid | ||
|Common Name=&&11-Hydroperoxy- | |Common Name=&&11-Hydroperoxy- 5,8,12,14 -eicosatetraenoic acid&& | ||
|Mass Spectra=GC-EI-MS(Me-ester; after reduction and TMS)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]][[Reference:Rabinovitch_H:Durand_J:Rigaud_M:Mendy_F:Breton_JC:,Lipids,1981,16,518|{{RelationTable/GetFirstAuthor|Reference:Rabinovitch_H:Durand_J:Rigaud_M:Mendy_F:Breton_JC:,Lipids,1981,16,518}}]], GC-EI-MS(Me-ester; after reduction and TBDMS)(114), GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]][[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]][[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]][[Reference:Mayer_B:Moser_R:Gleispach_H:Kukovetz_WR:,Biochim. Biophys. Acta,1986,875,641|{{RelationTable/GetFirstAuthor|Reference:Mayer_B:Moser_R:Gleispach_H:Kukovetz_WR:,Biochim. Biophys. Acta,1986,875,641}}]], GC-EI-MS(Me-ester; after reduction, hydrogenation and TBDMS) | |Mass Spectra=GC-EI-MS(Me-ester; after reduction and TMS)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]][[Reference:Rabinovitch_H:Durand_J:Rigaud_M:Mendy_F:Breton_JC:,Lipids,1981,16,518|{{RelationTable/GetFirstAuthor|Reference:Rabinovitch_H:Durand_J:Rigaud_M:Mendy_F:Breton_JC:,Lipids,1981,16,518}}]], GC-EI-MS(Me-ester; after reduction and TBDMS)(114), GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,595}}]][[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]][[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]][[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]][[Reference:Mayer_B:Moser_R:Gleispach_H:Kukovetz_WR:,Biochim. Biophys. Acta,1986,875,641|{{RelationTable/GetFirstAuthor|Reference:Mayer_B:Moser_R:Gleispach_H:Kukovetz_WR:,Biochim. Biophys. Acta,1986,875,641}}]], GC-EI-MS(Me-ester; after reduction, hydrogenation and TBDMS) | ||
|UV Spectra=UV[[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]] conjugated diene: lambda max=235nm, UV(Me-ester)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]] conjugated diene: lambda max=232.5nm, UV(Me-ester; after reduction)[[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]] conjugated trans, cis diene: lambda max=236nm, conjugated trans, trans diene: lambda max=232.5 | |UV Spectra=UV[[Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Logan_J:Kontoyiannidou_V:,J. Org. Chem.,1979,44,3177}}]] conjugated diene: lambda max=235nm, UV(Me-ester)[[Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587|{{RelationTable/GetFirstAuthor|Reference:Terao_J:Matsushita_S:,Agric. Biol. Chem.,1981,45,587}}]] conjugated diene: lambda max=232.5nm, UV(Me-ester; after reduction)[[Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058|{{RelationTable/GetFirstAuthor|Reference:Porter_NA:Wolf_RA:Yarbro_EM:Weenen_H:,Biochem. Biophys. Res. Commun.,1979,89,1058}}]] conjugated trans, cis diene: lambda max=236nm, conjugated trans, trans diene: lambda max=232.5 | ||
Revision as of 05:52, 4 November 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8082 |
| LipidMaps | LMFA03060041 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406HP10 |
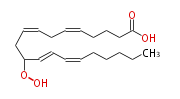
| 11-Hydroperoxy- 5,8,12,14 -eicosatetraenoic acid | |
|---|---|
| |
| Structural Information | |
| 11-Hydroperoxy- 5,8,12,14 -eicosatetraenoic acid | |
| |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC=CC=CC(OO)CC=CCC=CCCCC(O)=O)CCC |
| Physicochemical Information | |
| Autooxidation of arachidonic acid Terao_J et al. Yamagata_S et al. Porter_NA et al.. Oxidation of arachidonic acid by singlet-oxygen Terao_J et al. Porter_NA et al.. Reaction products between arachidonic acid and lipoxygenase from living cells(11-HPETE). | |
| 11-HPETE generated by 11-lipoxygenase is enzymatically converted to bioactive compounds such as leukotriene and HETE Spector_AA et al.. | |
| Isomerization of hydroperoxides : Both 5- and 15-hydroperoxide were generated predominantly by autooxidation or singlet-oxygen mediated oxidation Terao_J et al. Yamagata_S et al. Peers_KE et al.. However, the proportion of each isomer generated become almost equal by supplementation of hydrogen donor such as tocopherol Porter_NA et al. Terao_J et al. Peers_KE et al.. | |
| Spectral Information | |
| Mass Spectra | GC-EI-MS(Me-ester; after reduction and TMS) TeraoJet al. TeraoJet al. RabinovitchHet al., GC-EI-MS(Me-ester; after reduction and TBDMS)(114), GC-EI-MS(Me-ester; after reduction, hydrogenation and TMS) TeraoJet al. TeraoJet al. Porter_NA et al. Porter_NA et al. MayerBet al., GC-EI-MS(Me-ester; after reduction, hydrogenation and TBDMS) |
| UV Spectra | UV Porter_NA et al. conjugated diene: λ max=235nm, UV(Me-ester) TeraoJet al. conjugated diene: λ max=232.5nm, UV(Me-ester; after reduction) Porter_NA et al. conjugated trans, cis diene: λ max=236nm, conjugated trans, trans diene: λ max=232.5 |
| IR Spectra | IR Porter_NA et al.: conjugated trans, cis diene: 985, 950cm-1, OOH group: 3400cm-1, IR(Me-ester; after reduction) Porter_NA et al.conjugated trans, cis diene: 985, 950cm-1, conjugated trans, trans diene: 989cm-1 |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|