LBF20406LT02: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
|UV Spectra=METHANOL : 260(<FONT FACE="Symbol">e</FONT> 38,000), 270.5(<FONT FACE="Symbol">e</FONT> 50,000), 281(<FONT FACE="Symbol">e</FONT> 39,000)nm [[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] | |UV Spectra=METHANOL : 260(<FONT FACE="Symbol">e</FONT> 38,000), 270.5(<FONT FACE="Symbol">e</FONT> 50,000), 281(<FONT FACE="Symbol">e</FONT> 39,000)nm [[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, D<SUB><FONT SIZE=-1>2</FONT></SUB>O) : <FONT FACE="Symbol">d</FONT> 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) [[Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409|{{RelationTable/GetFirstAuthor|Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(250MHz, D<SUB><FONT SIZE=-1>2</FONT></SUB>O) : <FONT FACE="Symbol">d</FONT> 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) [[Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409|{{RelationTable/GetFirstAuthor|Reference:Merrer_YL:Gravier-Pelletier_C:Micas-Languin_D:Mestre_F:Durreault_A:Depezay_JC:,J. Org. Chem.,1989,54,2409}}]] | ||
|Source=Leukotriene B4 is produced by polymorphonuclear leukocytes and macrophages of various animal species upon various stimulations on the cells [[Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1|{{RelationTable/GetFirstAuthor|Reference:Samuelsson_B:Hammarstrom_S:,Vitam. Horm.,1982,39,1}}]][[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]];>. | |||
|Chemical Synthesis=[[Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Marfat_A:Goto_G:Brion_F:,J. Am. Chem. Soc.,1980,102,7984}}]];> {{Image200|XPR3101FT0001.gif}} | |||
|Metabolism=Arachidonic acid is metabolized to leukotrienen A4 with 5,6-epoxide by 5-lipoxygenases, and the product is further transformed to leukotrienee B4 by leukotriene A hydrolase [[Reference:Ford-Hutchinson_AW:Gresser_M:Young_RN:,Annu. Rev. Biochem.,1994,63,383|{{RelationTable/GetFirstAuthor|Reference:Ford-Hutchinson_AW:Gresser_M:Young_RN:,Annu. Rev. Biochem.,1994,63,383}}]];>. Leukotriene B4 is metabolized to lose its bioactivities either by <FONT FACE="Symbol">w</FONT>-oxidation [[Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355|{{RelationTable/GetFirstAuthor|Reference:Hammarstrom_S:,Annu. Rev. Biochem.,1983,52,355}}]];> or by leukotriene B4 12-hydroxydehydrogenase [[Reference:Yokomizo_T:Izumi_T:Takahashi_T:Kasama_T:Kobayashi_Y:Sato_F:Taketani_Y:Shimizu_T:,J. Biol. Chem.,1993,268,18128|{{RelationTable/GetFirstAuthor|Reference:Yokomizo_T:Izumi_T:Takahashi_T:Kasama_T:Kobayashi_Y:Sato_F:Taketani_Y:Shimizu_T:,J. Biol. Chem.,1993,268,18128}}]];>. | |||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 22:00, 24 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR3101 |
| LipidMaps | LMFA03020001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406LT02 |
| LEUKOTRIENE B4 | |
|---|---|
| |
| Structural Information | |
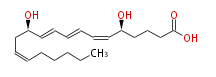
| 5 (S) ,12 (R) -Dihydroxyeicosa-6 (Z) ,8 (E) ,10 (E) ,14 (Z) -tetraenoic acid | |
| |
| Formula | C20H32O4 |
| Exact Mass | 336.23005951199997 |
| Average Mass | 336.46567999999996 |
| SMILES | C(CC=CC[C@H](C=CC=CC=C[C@H](CCCC(O)=O)O)O)CCC |
| Physicochemical Information | |
| METHANOL Corey_EJ et al. | |
| Leukotriene B4 is produced by polymorphonuclear leukocytes and macrophages of various animal species upon various stimulations on the cells Samuelsson_B et al. Hammarstrom_S ;>. | |
|
Corey_EJ et al.;> File:XPR3101FT0001.gif | |
| Arachidonic acid is metabolized to leukotrienen A4 with 5,6-epoxide by 5-lipoxygenases, and the product is further transformed to leukotrienee B4 by leukotriene A hydrolase Ford-Hutchinson_AW et al.;>. Leukotriene B4 is metabolized to lose its bioactivities either by w-oxidation Hammarstrom_S ;> or by leukotriene B4 12-hydroxydehydrogenase Yokomizo_T et al.;>. | |
| Spectral Information | |
| Mass Spectra | m/e 336, 319, 301 Yergey_JA et al. |
| UV Spectra | METHANOL : 260(e 38,000), 270.5(e 50,000), 281(e 39,000)nm Corey_EJ et al. |
| IR Spectra | |
| NMR Spectra | 1H-NMR(250MHz, D2O) : d 6.45(m, 1H, 8-CH), 6.15(m, 2H, 9,10-CH), 6.0(m, 1H, 7-CH), 5.65(m, 1H, 11-CH), 5.40(m, 1H, 15-CH), 5.25(m, 2H, 6,14-CH), 4.60(5-CH), 4.05(m, 1H, 12-CH), 2.15(m, 2H, 13-CH), 2.00(m, 1H, 2-CH), 1.85(m, 2H, 16-CH), 1.35-1.60(m, 4H, 3,4-CH), 1.00-1.25(m, 6H, 17,18,19-CH), 0.70(m, 3H, 20-CH) Merrer_YL et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|