LBF20406PH01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 11: | Line 11: | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (CDCl3) <FONT FACE="Symbol">d</FONT>5.34-5.42 (m, 8H), 3.98 (br s 2H), 3.46 (br s 2H), 2.77-2.81 (m, 6H), 2.26 (t, J=6.8Hz, 2H), 2.00-2.06 (m, 4H), 1.60-1.69 (m, 2H), 1.29-1.42 (m, 6H), 0.88 (t, J=6.9Hz,3H) [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H NMR (CDCl3) <FONT FACE="Symbol">d</FONT>5.34-5.42 (m, 8H), 3.98 (br s 2H), 3.46 (br s 2H), 2.77-2.81 (m, 6H), 2.26 (t, J=6.8Hz, 2H), 2.00-2.06 (m, 4H), 1.60-1.69 (m, 2H), 1.29-1.42 (m, 6H), 0.88 (t, J=6.9Hz,3H) [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|Source= | |Source= | ||
|Chemical Synthesis=This compound was synthesized from N-hydroxysuccinimide ester of arachidonic acid and O-phosphoethanolamine in 60% yield. [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | |Chemical Synthesis=This compound was synthesized from N-hydroxysuccinimide ester of arachidonic acid and O-phosphoethanolamine in 60% yield. [[Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659|{{RelationTable/GetFirstAuthor|Reference:Sheskin_T:Hanus_L:Slager_J:Vogel_Z:Mechoulam_R:,J. Med. Chem.,1997,40,659}}]] | ||
|Metabolism= | |Metabolism= | ||
}} | }} | ||
{{Lipid/Footer}} | {{Lipid/Footer}} | ||
Revision as of 11:55, 25 November 2009
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | XPR7016 |
| LipidMaps | LMFA08020002 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20406PH01 |
| anandamide 0-phosphate | |
|---|---|
| |
| Structural Information | |
| anandamide 0-phosphate | |
| |
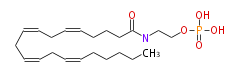
| Formula | C22H38NO5P |
| Exact Mass | 427.2487598409999 |
| Average Mass | 427.51462100000003 |
| SMILES | C(CCCC=CCC=CCC=CCC=CCCCCC)(NCCOP(O)(O)=O)=O |
| Physicochemical Information | |
| colorless oil Sheskin_T et al. | |
| This compound was synthesized from N-hydroxysuccinimide ester of arachidonic acid and O-phosphoethanolamine in 60% yield. Sheskin_T et al. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | 1H NMR (CDCl3) d5.34-5.42 (m, 8H), 3.98 (br s 2H), 3.46 (br s 2H), 2.77-2.81 (m, 6H), 2.26 (t, J=6.8Hz, 2H), 2.00-2.06 (m, 4H), 1.60-1.69 (m, 2H), 1.29-1.42 (m, 6H), 0.88 (t, J=6.9Hz,3H) SheskinTet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|