LBF20407LX01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
|LipidMaps=LMFA03040001 | |LipidMaps=LMFA03040001 | ||
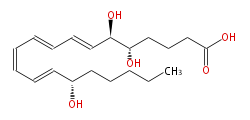
|SysName=5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid | |SysName=5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid | ||
|Common Name=&&lipoxin A_4 | |Common Name=&&lipoxin A_4&& | ||
|UV Spectra= lambda max: 302nm epsilon : 50,000 | |UV Spectra= lambda max: 302nm epsilon : 50,000 | ||
|Source=LXA4 is formed in vitro by a number of mechanisms from arachidonic acid, 15-HpETE, and leukotriene A4 (LTA4 ) [[Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401|{{RelationTable/GetFirstAuthor|Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401}}]][[Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335|{{RelationTable/GetFirstAuthor|Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335}}]]. | |Source=LXA4 is formed in vitro by a number of mechanisms from arachidonic acid, 15-HpETE, and leukotriene A4 (LTA4 ) [[Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401|{{RelationTable/GetFirstAuthor|Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401}}]][[Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335|{{RelationTable/GetFirstAuthor|Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335}}]]. | ||
Revision as of 21:00, 14 April 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8153 |
| LipidMaps | LMFA03040001 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20407LX01 |
| lipoxin A4 | |
|---|---|
| |
| Structural Information | |
| 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid | |
| |
| LXA4 | |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CCC(C=CC=CC=CC=CC(O)C(O)CCCC(O)=O)O)CC |
| Physicochemical Information | |
| LXA4 is formed in vitro by a number of mechanisms from arachidonic acid, 15-HpETE, and leukotriene A4 (LTA4 ) Edenius_C et al. Serhan_CN et al.. | |
| LXA is equipotent to LTB in inducing superoxide generation in human neutrophils (0.1 mu M) Serhan_CN et al.. LXA4 is associated with several other biological functions including leukocyte activation, chemotaxis promotion, natural killer cell inhibition, and monocyte migration and adhesion Serhan_CN et al. Ramstedt_U et al. Maddox_JF et al.. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 302nm ε : 50,000 |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|