LBF20407LX01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|SysName=(5S,6R,15S) -Trihydroxy- (trans-7,trans-9,cis-11,trans-13) -eicosatetraenoic acid | |SysName=(5S,6R,15S) -Trihydroxy- (trans-7,trans-9,cis-11,trans-13) -eicosatetraenoic acid | ||
|Common Name=&&Lipoxin A_4&&(5S,6R,15S) -Trihydroxy- (7E,9E,11Z,13E) -eicosatetraenoic acid&& | |Common Name=&&Lipoxin A_4&&(5S,6R,15S) -Trihydroxy- (7E,9E,11Z,13E) -eicosatetraenoic acid&& | ||
|UV Spectra=lambda max: 302nm epsilon : 50,000 | |UV Spectra=lambda max: 302nm epsilon : 50,000, lambda MeOHmax: 287,301 (epsilon 50,000) , 316nm [[Reference:Adams_J:Fitzsimmons_BJ:Girard_Y:Leblanc_Y:Evans_JF:Rokach_J:,J._Am._Chem._Soc.,1985,107,464|{{RelationTable/GetFirstAuthor|Reference:Adams_J:Fitzsimmons_BJ:Girard_Y:Leblanc_Y:Evans_JF:Rokach_J:,J._Am._Chem._Soc.,1985,107,464}}]], [[Reference:Serhan_CN:Nicolaou_KC:Webber_SE:Veale_CA:Dahlen_SE:Puustinen_TJ:Samuelsson_B:,J._Biol._Chem.,1986,261,16340|{{RelationTable/GetFirstAuthor|Reference:Serhan_CN:Nicolaou_KC:Webber_SE:Veale_CA:Dahlen_SE:Puustinen_TJ:Samuelsson_B:,J._Biol._Chem.,1986,261,16340}}]] | ||
|Source=LXA4 is formed in vitro by a number of mechanisms from arachidonic acid, 15-HpETE, and leukotriene A4 (LTA4 ) [[Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401|{{RelationTable/GetFirstAuthor|Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401}}]][[Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335|{{RelationTable/GetFirstAuthor|Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335}}]]. | |Source=LXA4 is formed in vitro by a number of mechanisms from arachidonic acid, 15-HpETE, and leukotriene A4 (LTA4 ) [[Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401|{{RelationTable/GetFirstAuthor|Reference:Edenius_C:Stenke_L:Lindgren_JA:,Eur. J. Biochem.,1991,199,401}}]][[Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335|{{RelationTable/GetFirstAuthor|Reference:Serhan_CN:Hamberg_M:Samuelsson_B:,Proc. Natl. Acad. Sci. U. S. A.,1984,81,5335}}]]. | ||
|Chemical Synthesis= | |Chemical Synthesis= | ||
Revision as of 03:34, 20 January 2014
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA8153 |
| LipidMaps | LMFA03040001 |
| CAS | 89663-86-5 |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20407LX01 |
| Lipoxin A4 | |
|---|---|
| |
| Structural Information | |
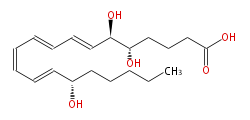
| (5S,6R,15S) -Trihydroxy- (trans-7,trans-9,cis-11,trans-13) -eicosatetraenoic acid | |
| |
| LXA4 | |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CCC(C=CC=CC=CC=CC(O)C(O)CCCC(O)=O)O)CC |
| Physicochemical Information | |
| LXA4 is formed in vitro by a number of mechanisms from arachidonic acid, 15-HpETE, and leukotriene A4 (LTA4 ) Edenius_C et al. Serhan_CN et al.. | |
| LXA is equipotent to LTB in inducing superoxide generation in human neutrophils (0.1 mu M) Serhan_CN et al.. LXA4 is associated with several other biological functions including leukocyte activation, chemotaxis promotion, natural killer cell inhibition, and monocyte migration and adhesion Serhan_CN et al. Ramstedt_U et al. Maddox_JF et al.. Lipoxin A4 is either vasoconstrictive or vasodilatory, and has immunoregulatory activities blocking the cytotoxic action of NK cell, inhibiting adherence and chemotaxis of leukocytes induced by FMLP, PAF or leukotriene B4 Serhan_CN . | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | λ max: 302nm ε : 50,000, λ MeOHmax: 287,301 (ε 50,000) , 316nm AdamsJet al., Serhan_CN et al. |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|