LBF08106SC01: Difference between revisions
No edit summary |
No edit summary |
||
| (19 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Lipid/Header}} | |||
{{Hierarchy|{{PAGENAME}}}} | {{Hierarchy|{{PAGENAME}}}} | ||
| Line 5: | Line 7: | ||
|LipidMaps=LMFA01030017 | |LipidMaps=LMFA01030017 | ||
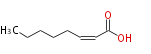
|SysName=cis-2-Octenoic acid | |SysName=cis-2-Octenoic acid | ||
|Common Name=&& | |Common Name=&&cis-alpha-Octenoic acid&&2Z-Octenoic acid&&3-n-Amylacrylic acid&& | ||
|Boiling Point=154°C at 22mmHg | |Boiling Point=154°C at 22mmHg | ||
|Density= | |Density=d^{20}_4 0.9807 | ||
| | |Refractive=1.4587 at 20°C | ||
|Solubility=[[Reference:Bachman_GB:,J. Am. Chem. Soc.,1923,55,4279|{{RelationTable/GetFirstAuthor|Reference:Bachman_GB:,J. Am. Chem. Soc.,1923,55,4279}}]][[Reference:Knight_JA:Diamond_JH:,J. Org. Chem.,1959,24,400|{{RelationTable/GetFirstAuthor|Reference:Knight_JA:Diamond_JH:,J. Org. Chem.,1959,24,400}}]] | |Solubility=[[Reference:Bachman_GB:,J. Am. Chem. Soc.,1923,55,4279|{{RelationTable/GetFirstAuthor|Reference:Bachman_GB:,J. Am. Chem. Soc.,1923,55,4279}}]]<!--0157-->[[Reference:Knight_JA:Diamond_JH:,J. Org. Chem.,1959,24,400|{{RelationTable/GetFirstAuthor|Reference:Knight_JA:Diamond_JH:,J. Org. Chem.,1959,24,400}}]] | ||
|Source= | |||
|Chemical Synthesis=Synthesized by condensation of caprylic aldehyde with malonic acid in the presence of pyridine. | |||
|Metabolism= | |||
|Symbol=C8:1 | |||
}} | }} | ||
{{Lipid/Footer}} | |||
Latest revision as of 08:58, 4 October 2010
| LipidBank Top (トップ) |
Fatty acid (脂肪酸) |
Glycerolipid (グリセロ脂質) |
Sphingolipid (スフィンゴ脂質) |
Journals (雑誌一覧) |
How to edit (ページの書き方) |
| IDs and Links | |
|---|---|
| LipidBank | DFA0056 |
| LipidMaps | LMFA01030017 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF08106SC01 |
| cis-α-Octenoic acid | |
|---|---|
| |
| Structural Information | |
| cis-2-Octenoic acid | |
| |
| C8:1 | |
| Formula | C8H14O2 |
| Exact Mass | 142.09937969199999 |
| Average Mass | 142.19556 |
| SMILES | CCCCCC=CC(O)=O |
| Physicochemical Information | |
| 154°C at 22mmHg | |
| d20 4 0.9807 | |
| 1.4587 at 20°C | |
| Bachman_GB Knight_JA et al. | |
| Synthesized by condensation of caprylic aldehyde with malonic acid in the presence of pyridine. | |
| Spectral Information | |
| Mass Spectra | |
| UV Spectra | |
| IR Spectra | |
| NMR Spectra | |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|