LBF20107PG01: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
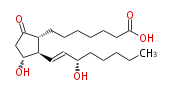
|SysName=7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -5-oxocyclopentan-1 (R) -yl ] -heptanoic acid / (8R,11R,12R,13E,15S) -11,15-Dihydroxy-9-oxo-13-prostenoic acid | |SysName=7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -5-oxocyclopentan-1 (R) -yl ] -heptanoic acid / (8R,11R,12R,13E,15S) -11,15-Dihydroxy-9-oxo-13-prostenoic acid | ||
|Common Name=&&PROSTAGLANDIN E1&& | |Common Name=&&PROSTAGLANDIN E1&& | ||
|Melting Point=115-117°C | |Melting Point=115-117°C | ||
|Reflactive=[<FONT FACE="Symbol">a</FONT>]<SUB><FONT SIZE=-1>5</FONT></SUB><SUB><FONT SIZE=-1>7</FONT></SUB><SUB><FONT SIZE=-1>8</FONT></SUB>=-61.6°(C=0.56, TETRAHYDROFURAN) | |Reflactive=[<FONT FACE="Symbol">a</FONT>]<SUB><FONT SIZE=-1>5</FONT></SUB><SUB><FONT SIZE=-1>7</FONT></SUB><SUB><FONT SIZE=-1>8</FONT></SUB>=-61.6°(C=0.56, TETRAHYDROFURAN) [[Reference:Corey_EJ:Vlattas_I:Harding_K:,J. Am. Chem. Soc.,1969,91,535|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Vlattas_I:Harding_K:,J. Am. Chem. Soc.,1969,91,535}}]] | ||
|Solubility=DIETHYL ETHER, ETHYL ACETATE | |Solubility=DIETHYL ETHER, ETHYL ACETATE , METHANOL [[Reference:Struijk_MCB:Beerthuis_RK:Pabon_HJJ:Van_Dorp_DA:,Recueil Travaux Quimiq Pays Bas,1966,85,1233|{{RelationTable/GetFirstAuthor|Reference:Struijk_MCB:Beerthuis_RK:Pabon_HJJ:Van_Dorp_DA:,Recueil Travaux Quimiq Pays Bas,1966,85,1233}}]], TETRAHYDROFURAN [[Reference:Corey_EJ:Vlattas_I:Harding_K:,J. Am. Chem. Soc.,1969,91,535|{{RelationTable/GetFirstAuthor|Reference:Corey_EJ:Vlattas_I:Harding_K:,J. Am. Chem. Soc.,1969,91,535}}]] | ||
|Mass Spectra=METHYL ESTER ; m/e 350, 332, 319, 301, 279 | |Mass Spectra=METHYL ESTER ; m/e 350, 332, 319, 301, 279 [[Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257|{{RelationTable/GetFirstAuthor|Reference:Hamberg_M:Samuelsson_B:,J. Biol. Chem.,1966,241,257}}]] | ||
|IR Spectra=METHYL ESTER ; <FONT FACE="Symbol">n</FONT> 1726, 1717sh, 1699, 980 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=METHYL ESTER ; <FONT FACE="Symbol">n</FONT> 1726, 1717sh, 1699, 980 cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Hayashi_M:Miyamoto_T:,Metabolism and Disease (Taisha),1975,12,1461|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Miyamoto_T:,Metabolism and Disease (Taisha),1975,12,1461}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>+DMSO-d<SUB><FONT SIZE=-1>6</FONT></SUB>,TMS) : <FONT FACE="Symbol">d</FONT> 5.70-5.51(m, 2H), 4.14-3.86(m, 2H), 2.72(d,d, 1H) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>+DMSO-d<SUB><FONT SIZE=-1>6</FONT></SUB>,TMS) : <FONT FACE="Symbol">d</FONT> 5.70-5.51(m, 2H), 4.14-3.86(m, 2H), 2.72(d,d, 1H)[[Reference:Hayashi_M:Miyamoto_T:,Metabolism and Disease (Taisha),1975,12,1461|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Miyamoto_T:,Metabolism and Disease (Taisha),1975,12,1461}}]]. <SUP><FONT SIZE=-1>1</FONT></SUP><SUP><FONT SIZE=-1>3</FONT></SUP>C-NMR(CHCl<SUB><FONT SIZE=-1>3</FONT></SUB>-CH<SUB><FONT SIZE=-1>3</FONT></SUB>OH, TMS) : <FONT FACE="Symbol">d</FONT> 215.2(C-9), 176.7(C-1), 136.6(C-14), 131.9(C-13), 72.9(C-15), 71.6(C-11), 54.6(C-8), 54.2(C-12), 45.9(C-10), 36.9(C-16), 33.8(C-2), 31.5(C-18), 29.0(C-4), 28.6(C-5), 27.4(C-7), 26.3(C-6), 25.0(C-17), 24.5(C-3), 22.5(C-19), 13.8(C-20) [[Reference:Lukacs_G:Piriou_F:Gero_SD:,Tetrah. Lett.,1973,,515|{{RelationTable/GetFirstAuthor|Reference:Lukacs_G:Piriou_F:Gero_SD:,Tetrah. Lett.,1973,,515}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1400 |
| LipidMaps | LMFA03010134 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20107PG01 |
| PROSTAGLANDIN E1 | |
|---|---|
| |
| Structural Information | |
| 7- [ 3 (R) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -5-oxocyclopentan-1 (R) -yl ] -heptanoic acid / (8R,11R,12R,13E,15S) -11,15-Dihydroxy-9-oxo-13-prostenoic acid | |
| |
| Formula | C20H34O5 |
| Exact Mass | 354.240624198 |
| Average Mass | 354.48096000000004 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CCCCCCC(O)=O)[C@@H](CC1=O)O)CC |
| Physicochemical Information | |
| 115-117°C | |
| DIETHYL ETHER, ETHYL ACETATE , METHANOL Struijk_MCB et al., TETRAHYDROFURAN Corey_EJ et al. | |
| Spectral Information | |
| Mass Spectra | METHYL ESTER ; m/e 350, 332, 319, 301, 279 HambergMet al. |
| UV Spectra | |
| IR Spectra | METHYL ESTER ; n 1726, 1717sh, 1699, 980 cm-1 HayashiMet al. |
| NMR Spectra | 1H-NMR(CDCl3+DMSO-d6,TMS) : d 5.70-5.51(m, 2H), 4.14-3.86(m, 2H), 2.72(d,d, 1H) HayashiMet al.. 13C-NMR(CHCl3-CH3OH, TMS) : d 215.2(C-9), 176.7(C-1), 136.6(C-14), 131.9(C-13), 72.9(C-15), 71.6(C-11), 54.6(C-8), 54.2(C-12), 45.9(C-10), 36.9(C-16), 33.8(C-2), 31.5(C-18), 29.0(C-4), 28.6(C-5), 27.4(C-7), 26.3(C-6), 25.0(C-17), 24.5(C-3), 22.5(C-19), 13.8(C-20) LukacsGet al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|