LBF20207PG22: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|SysName=7- [ 5 (S) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -3-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |SysName=7- [ 5 (S) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -3-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | ||
|Common Name=&&PROSTAGLANDIN D2&& | |Common Name=&&PROSTAGLANDIN D2&& | ||
|Melting Point=68°C | |Melting Point=68°C [[Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115}}]] | ||
|Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>20</sup>=9° (C=2.11,THF) | |Reflactive=[<FONT FACE="Symbol">a</FONT>]X<sub>D</sub><sup>20</sup>=9° (C=2.11,THF) [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | ||
|Solubility=ETHYL ACETATE,THF,CHLOROFORM | |Solubility=ETHYL ACETATE,THF,CHLOROFORM [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | ||
|Mass Spectra=m/e 334, 316, 246, 245, 191, 190, 161, 55 | |Mass Spectra=m/e 334, 316, 246, 245, 191, 190, 161, 55 [[Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625|{{RelationTable/GetFirstAuthor|Reference:Ogawa_Y:Nunomoto_M:Shibasaki_M:,J. Org. Chem.,1986,51,1625}}]] | ||
|IR Spectra=KBr : 3400-2500, 1740, 1700, 975cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> | |IR Spectra=KBr : 3400-2500, 1740, 1700, 975cm<SUP><FONT SIZE=-1>-</FONT></SUP><SUP><FONT SIZE=-1>1</FONT></SUP> [[Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115|{{RelationTable/GetFirstAuthor|Reference:Hayashi_M:Tanouchi_T:,J. Org. Chem.,1973,38,2115}}]] | ||
|NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(270MHz, CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 5.63(dd,J=7 & 16Hz,1H,14-CH), 5.43(dd,J=16 & 8.5Hz,1H,13-CH), 4.47(m, 1H, 9-CH), 4.09(bq, J=7Hz, 1H, 15-CH), 2.87(dd, J=12 & 8.5Hz, 1H, 12-CH), 2.45(m, 2H, 10-CH2), 1.95(bm, 1H, 8-CH) | |NMR Spectra=<SUP><FONT SIZE=-1>1</FONT></SUP>H-NMR(270MHz, CDCl<SUB><FONT SIZE=-1>3</FONT></SUB>) : <FONT FACE="Symbol">d</FONT> 5.63(dd,J=7 & 16Hz,1H,14-CH), 5.43(dd,J=16 & 8.5Hz,1H,13-CH), 4.47(m, 1H, 9-CH), 4.09(bq, J=7Hz, 1H, 15-CH), 2.87(dd, J=12 & 8.5Hz, 1H, 12-CH), 2.45(m, 2H, 10-CH2), 1.95(bm, 1H, 8-CH) [[Reference:Jenny_EF:Schaeublin_P:,Tetrah. Lett.,1974,,2235|{{RelationTable/GetFirstAuthor|Reference:Jenny_EF:Schaeublin_P:,Tetrah. Lett.,1974,,2235}}]] | ||
}} | }} | ||
Revision as of 00:00, 12 December 2008
| IDs and Links | |
|---|---|
| LipidBank | XPR1301 |
| LipidMaps | LMFA03010004 |
| CAS | |
| KEGG | {{{KEGG}}} |
| KNApSAcK | {{{KNApSAcK}}} |
| mol | LBF20207PG22 |
| PROSTAGLANDIN D2 | |
|---|---|
| |
| Structural Information | |
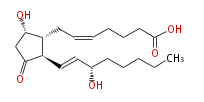
| 7- [ 5 (S) -Hydroxy-2 (R) - (3 (S) -hydroxy-1 (E) -octenyl) -3-oxocyclopentan-1 (R) -yl ] -5 (Z) -heptenoic acid | |
| |
| Formula | C20H32O5 |
| Exact Mass | 352.224974134 |
| Average Mass | 352.46508 |
| SMILES | C(CC[C@@H](O)C=C[C@H]([C@H]1CC=CCCCC(O)=O)C(C[C@@H]1O)=O)CC |
| Physicochemical Information | |
| 68°C Hayashi_M et al. | |
| ETHYL ACETATE,THF,CHLOROFORM OgawaYet al. | |
| Spectral Information | |
| Mass Spectra | m/e 334, 316, 246, 245, 191, 190, 161, 55 OgawaYet al. |
| UV Spectra | |
| IR Spectra | KBr : 3400-2500, 1740, 1700, 975cm-1 HayashiMet al. |
| NMR Spectra | 1H-NMR(270MHz, CDCl3) : d 5.63(dd,J=7 & 16Hz,1H,14-CH), 5.43(dd,J=16 & 8.5Hz,1H,13-CH), 4.47(m, 1H, 9-CH), 4.09(bq, J=7Hz, 1H, 15-CH), 2.87(dd, J=12 & 8.5Hz, 1H, 12-CH), 2.45(m, 2H, 10-CH2), 1.95(bm, 1H, 8-CH) Jenny_EF et al. |
| Other Spectra | |
| Chromatograms | |
| Reported Metabolites, References | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|